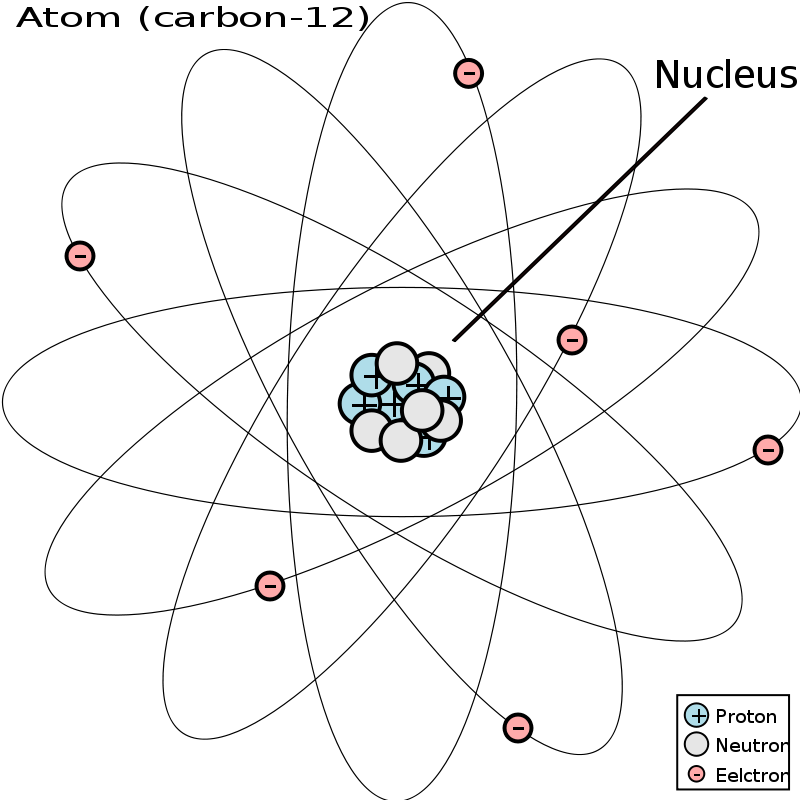
A Carbon Atom Is Most Likely To Form
A Carbon Atom Is Most Likely To Form - The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also with itself. Methane, (\(\ce{ch4}\), is a single carbon atom covalently bonded to four. Web carbon most often forms a covalent bond with other atoms. Web ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms. The majority of their bonds are nonpolar. Web science chemistry chemistry questions and answers which atom is most likely to form a polar covalent bond with carbon? Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Web a carbon atom is most likely to form what kind of bond(s) with other atoms? A carbon atom is most likely to form covalent bond (s) with other atoms. O ionic o hydrogen covalent o ionic bonds, covalent bonds, and hydrogen bonds submit request.
Why are hydrocarbons insoluble in water? 100% (1 rating) a carbon atom is most likely to form covalent bonds with other atoms. The majority of their bonds are nonpolar. Web science biology biology questions and answers 11. Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Ionic bonds, covalent bonds, and hydrogen bonds the correct answer is covalent. Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Carbon atom is a group 14 element and its atomic number is 6. Web carbon most often forms a covalent bond with other atoms. Web a carbon atom is most likely to form what kind of bond (s) with other atoms?
If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Web a carbon atom is most likely to form what kind of bond (s) with other atoms? This is because they tend to share electrons as carbon typically bonds with elements that have a similar. Why are hydrocarbons insoluble in water? The majority of their bonds are nonpolar. 100% (1 rating) a carbon atom is most likely to form covalent bonds with other atoms. Methane, (\(\ce{ch4}\), is a single carbon atom covalently bonded to four. Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Web science biology biology questions and answers a carbon atom is most likely to form which of the following bonds with other atoms?
Inside Atoms worksheet from EdPlace
Web ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms. Web which atom is most likely to form a polar covalent bond with carbon? Web science biology biology questions and answers 11. A carbon atom is most likely to form covalent bond (s) with other atoms. Web a carbon atom is most likely to form what kind.
What Causes Hot Things to Glow? Let's Talk Science
4/28/2022 wiki user ∙ 13y ago study now see answer (1) best answer copy. A) ionic b) hydrogen c) covalent d) ionic bonds,. Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Which atom is most likely to. Web which atom is most likely to form a polar covalent bond with carbon?
Does The Difference In Structure Make Graphite Soft But Diamond Hard?
Web which atom is most likely to form a polar covalent bond with carbon? The majority of their bonds are nonpolar. Web ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms. A) ionic b) hydrogen c) covalent d) ionic bonds,. Why are hydrocarbons insoluble in water?
The importance of carbon The Statesman
A) ionic b) hydrogen c) covalent d) ionic bonds,. Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Web science biology biology questions and answers 11. Ionic bonds, covalent bonds, and hydrogen bonds the correct answer is covalent. Web a carbon atom is most likely to form what kind of bond(s) with.
Carbon Atoms High Resolution Stock Photography and Images Alamy
The majority of their bonds are nonpolar. Web which atom is most likely to form a polar covalent bond with carbon? Methane, (\(\ce{ch4}\), is a single carbon atom covalently bonded to four. If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Ionic bonds, covalent bonds, and hydrogen bonds the correct answer is.
CBSE Papers, Questions, Answers, MCQ December 2012
If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Web a carbon atom is most likely to form what kind of bond(s) with other atoms? The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also with itself. Web.
A carbon atom is most likely to form what kind of bond(s) with other
Why are hydrocarbons insoluble in water? Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Web which atom is most likely to form a polar covalent bond with carbon? Web carbon most often forms a covalent bond.
How do the bonding properties of carbon atoms allow for the large
The majority of their bonds are nonpolar. 100% (1 rating) a carbon atom is most likely to form covalent bonds with other atoms. A) ionic b) hydrogen c) covalent d) ionic bonds,. Methane, (\(\ce{ch4}\), is a single carbon atom covalently bonded to four. Web ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms.
Visualizing Chemistry Homework 1/10/11
Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Web science biology biology questions and answers a carbon atom is most likely to form which of the following bonds with other atoms? 4/28/2022 wiki user ∙ 13y ago study now see answer (1) best answer copy. A carbon atom is most likely.
Diagram Of A Carbon Atom HighRes Stock Photo Getty Images
Web carbon most often forms a covalent bond with other atoms. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also with itself. If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Web science biology biology questions and.
The Majority Of Their Bonds Are Nonpolar.
Web science chemistry chemistry questions and answers which atom is most likely to form a polar covalent bond with carbon? Web science biology biology questions and answers a carbon atom is most likely to form which of the following bonds with other atoms? Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Web a carbon atom is most likely to form what kind of bond (s) with other atoms?
This Is Because They Tend To Share Electrons As Carbon Typically Bonds With Elements That Have A Similar.
Methane, (\(\ce{ch4}\), is a single carbon atom covalently bonded to four. A) ionic b) hydrogen c) covalent d) ionic bonds,. Web carbon most often forms a covalent bond with other atoms. Web science biology biology questions and answers 11.
Web A Carbon Atom Is Most Likely To Form What Kind Of Bond (S) With Other Atoms?
Web a carbon atom is most likely to form what kind of bond (s) with other atoms? A carbon atom is most likely to form covalent bond (s) with other atoms. 4/28/2022 wiki user ∙ 13y ago study now see answer (1) best answer copy. Web a carbon atom is most likely to form what kind of bond with other atoms?
Web Which Atom Is Most Likely To Form A Polar Covalent Bond With Carbon?
O ionic o hydrogen covalent o ionic bonds, covalent bonds, and hydrogen bonds submit request. Web a carbon atom is most likely to form what kind of bond(s) with other atoms? If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Carbon atom is a group 14 element and its atomic number is 6.