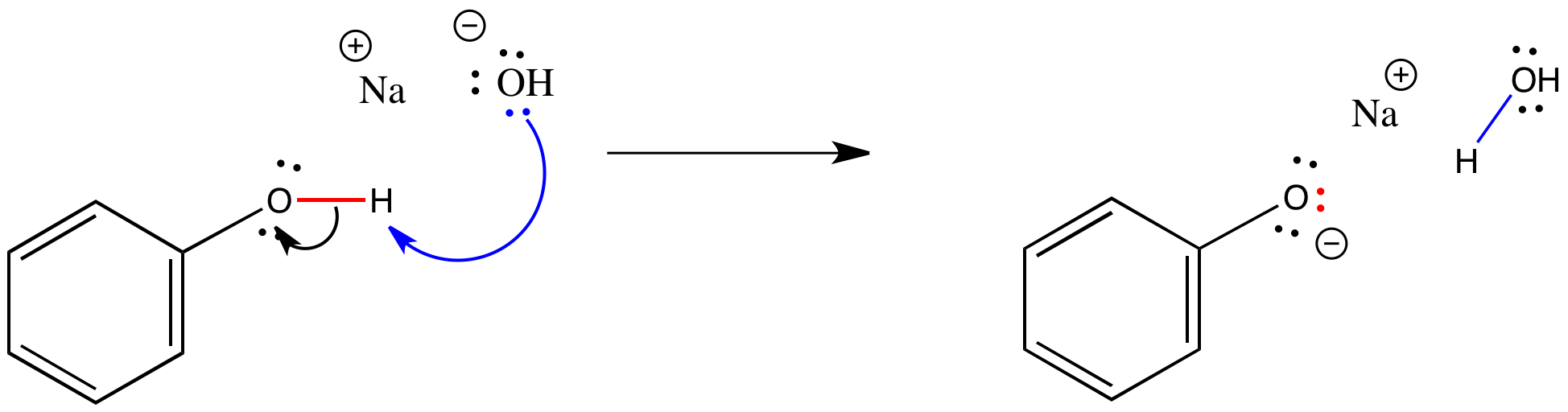
Acids React With Bases To Form
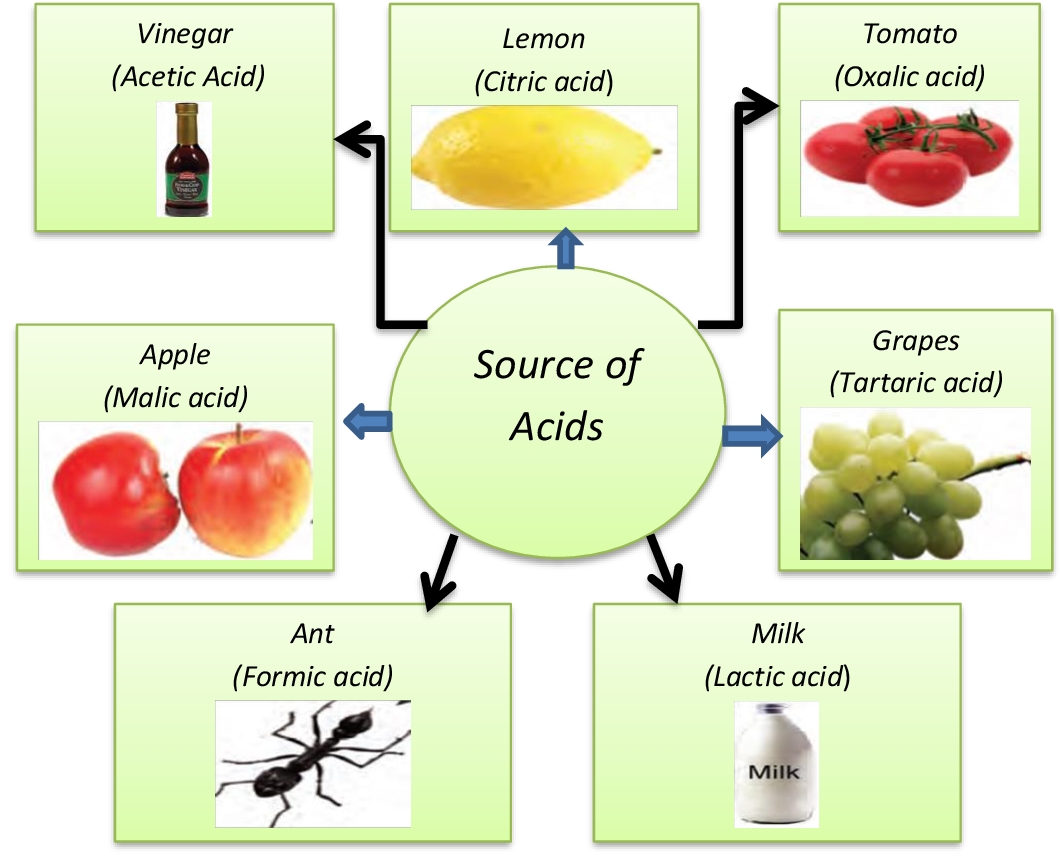
Acids React With Bases To Form - Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas. Web acids react with metals, bases and carbonates to produce salts. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Web an acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form. The neutralization of a strong acid and strong base has a ph equal to 7. It is a strong acid. Part of chemistry (single science) chemistry of. Taste sour (don't taste them!)—the word 'acid' comes from the latin acere, which means 'sour'. Sulfuric acid + copper(ii) oxide → copper(ii) sulfate + water This could be because it is a hydroxide salt, like naoh, or.
Sulfuric acid + copper(ii) oxide → copper(ii) sulfate + water Web acid reacts with bases to form __________. Web acids react with bases to produce a salt compound and water. An ionic compound formed from the positive ion of the. Neutralisation is the reaction between an acid and a base. It is a strong acid. The neutralization of a strong acid and strong base has a ph equal to 7. As discussed in chapter 7, metals that are more active. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid.
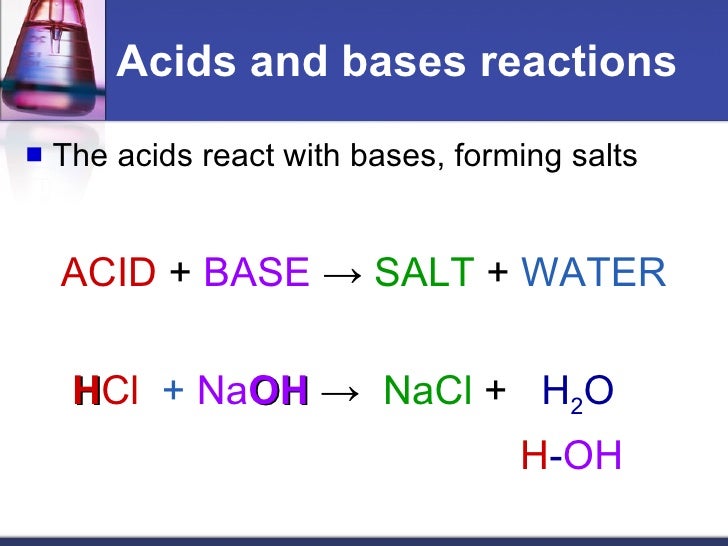
An ionic compound formed from the positive ion of the. The neutralization of a strong acid and strong base has a ph equal to 7. It is a strong acid. When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. Web acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus paper), reacts with some metals (e.g., iron) to. Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas. Acid + base → salt + water. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Taste sour (don't taste them!)—the word 'acid' comes from the latin acere, which means 'sour'. Web when an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt.
Acids Bases
Web terms in this set (11) acids react with bases to form a ___________ and a ____________. Web acid reacts with bases to form __________. Web acids react with bases to produce a salt compound and water. Ammonia is not a strong base. Web acid reacts with metals to form salt and hydogen gas is also released.
CHAPTER 2 ACIDS BASES SALTS 1 Acknowledgment Images
A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid. Web an acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with.
Solved Carboxylic acids react with bases such as NaOH to
Web a base is an electrolyte (strong or weak) that produces hydroxide ions when dissolved in water solution. A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid. Web answer acids and bases react with metals acids react with.
Acids and Bases 9 Properties, Useful Reaction & Examples
Part of chemistry (single science) chemistry of. Web acids react with bases to produce a salt compound and water. Acid + metals → salt + hydrogen gas. When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. Web solution a neutralization reaction is when an acid and a base react to form water and a salt.
The Chemistry of Acids and Bases Presentation Chemistry
Web acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus paper), reacts with some metals (e.g., iron) to. Web acid reacts with metals to form salt and hydogen gas is also released. Web when an acid and a base are placed together, they react to neutralize the acid and base.
AcidBase Extraction Chemistry LibreTexts
Acid + base → salt + water. This could be because it is a hydroxide salt, like naoh, or. As discussed in chapter 7, metals that are more active. An ionic compound formed from the positive ion of the. Acids change litmus (a blue vegetable.
Lecture 19.1a Acid/Base Properties
Ammonia is not a strong base. Web acids react with bases to produce a salt compound and water. Acids react with bases to form a salt and water. When zinc (zn) reacts with dilute sulphuric acid. Web solution a neutralization reaction is when an acid and a base react to form water and a salt.
Acids, bases and salts CPD RSC Education
This could be because it is a hydroxide salt, like naoh, or. Neutralisation is the reaction between an acid and a base. Web a base is an electrolyte (strong or weak) that produces hydroxide ions when dissolved in water solution. Acids change litmus (a blue vegetable. It is a strong acid.
Properties and Uses of Acids Lesson Plan Coaches
Web a base is an electrolyte (strong or weak) that produces hydroxide ions when dissolved in water solution. Acids react with bases to form a salt and water. When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. When zinc (zn) reacts with dilute sulphuric acid. Taste sour (don't taste them!)—the word 'acid' comes from the latin.
How do acids and bases react with each other Neutralization Reaction
Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas. Web acids react with bases to produce a salt compound and water. Acid + metals → salt + hydrogen gas. Neutralisation is the reaction between an acid and a base. Web acid, any substance that in water solution tastes sour,.
The Neutralization Of A Strong Acid And Strong Base Has A Ph Equal To 7.
Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Acids react with bases to form a salt and water. Web terms in this set (11) acids react with bases to form a ___________ and a ____________. Web acids react with metals, bases and carbonates to produce salts.
Web A Base Is An Electrolyte (Strong Or Weak) That Produces Hydroxide Ions When Dissolved In Water Solution.
An ionic compound formed from the positive ion of the. Web acid reacts with metals to form salt and hydogen gas is also released. Ammonia is not a strong base. This could be because it is a hydroxide salt, like naoh, or.
Web Solution A Neutralization Reaction Is When An Acid And A Base React To Form Water And A Salt.
A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid. As discussed in chapter 7, metals that are more active. Acid + base → salt + water. Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas.
Web Acids React With Bases To Produce A Salt Compound And Water.
When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. Web when an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. Acid + metals → salt + hydrogen gas. Web in fact, this reaction is so iconic that we define a base as any compound that undergoes neutralisation reaction with acids.
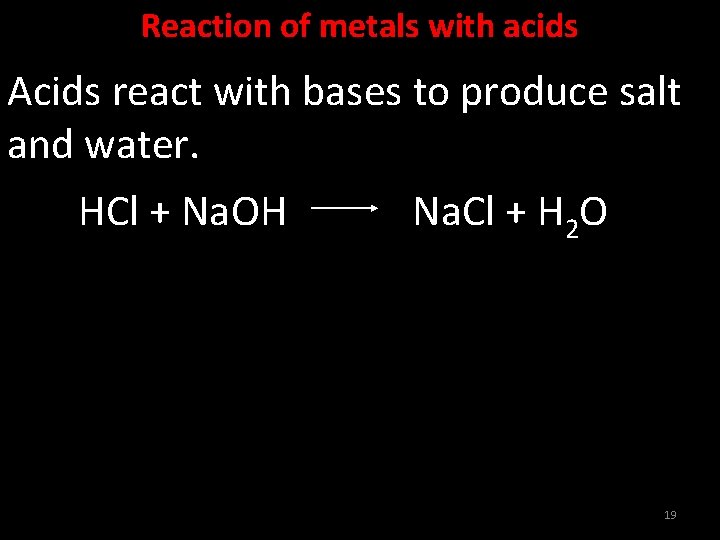

.PNG)