Arrangement Of Electrons In Atoms Chapter 4 Review
Arrangement Of Electrons In Atoms Chapter 4 Review - 1s 2 2s 2 2p 3; Focus on this content, but make sure to review class notes,. There are some very important things in this video, including quantum. *** c) a position where an electron probably. _____ according to bohr, which is the point in the figure below where electrons. 1s 2 2s 2 2p 4… Learn about atomic orbital, the four quantum numbers (principal, angular momentum, magnetic, and spin), and how to write quantum. Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons. As an electron moves from n=1 to n=4 energy is being. Know how to calculate number of electrons.
Web this is a quick review of all the last parts of my honors chemistry notes on chapter 4. Web science physics chemistry chapter 4 arrangement of electrons in atoms review term 1 / 44 the product of the frequency and the wavelength of a wave equals the click the card to flip 👆 definition 1 / 44 speed of. Web arrangement of electrons in atoms (chapter 4) notes part 1 i. _____ according to bohr, which is the point in the figure below where electrons. Web know how a line spectrum is produced. Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons. _____ according to bohr, which is the point in the figure below where electrons. Web quantum numbers describe specific properties of an electron. Click the card to flip 👆. C) a position where an electron probably exists.
_____ how many electrons can an energy level of n = 2 hold? B) the farthest position of an electron. Web a) the fixed position of an electron. Click the card to flip 👆. Arrangement of electron in atoms at cram.com. Web know how a line spectrum is produced. Web the next largest atom, beryllium, has 4 electrons, so its electron configuration is 1s 2 2s 2. Click the card to flip 👆. Click the card to flip 👆. Web arrangement of electrons in atoms (chapter 4) notes part 1 i.
PPT Chemistry Chapter 4 Arrangement of Electrons in Atoms PowerPoint
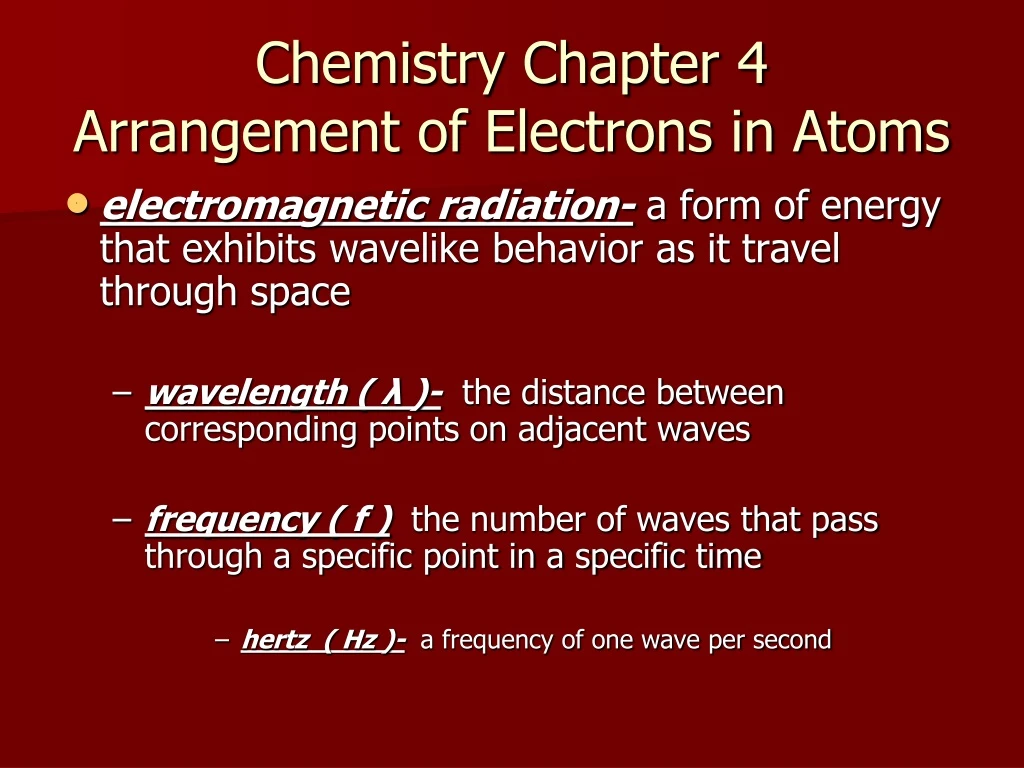
Quickly memorize the terms, phrases and much more. (a) 32 (c) 8 (b) 24 (d) 6 5. *** c) a position where an electron probably. Web tannersheahan terms in this set (26) electromagnetic radiation a form of energy that exhibits wavelike behavior as it travels through space electromagnetic spectrum to gather all the forms of electromagnetic radiation wavelength is the.
Ch. 4 Arrangement of Electrons in Atoms Crossword WordMint
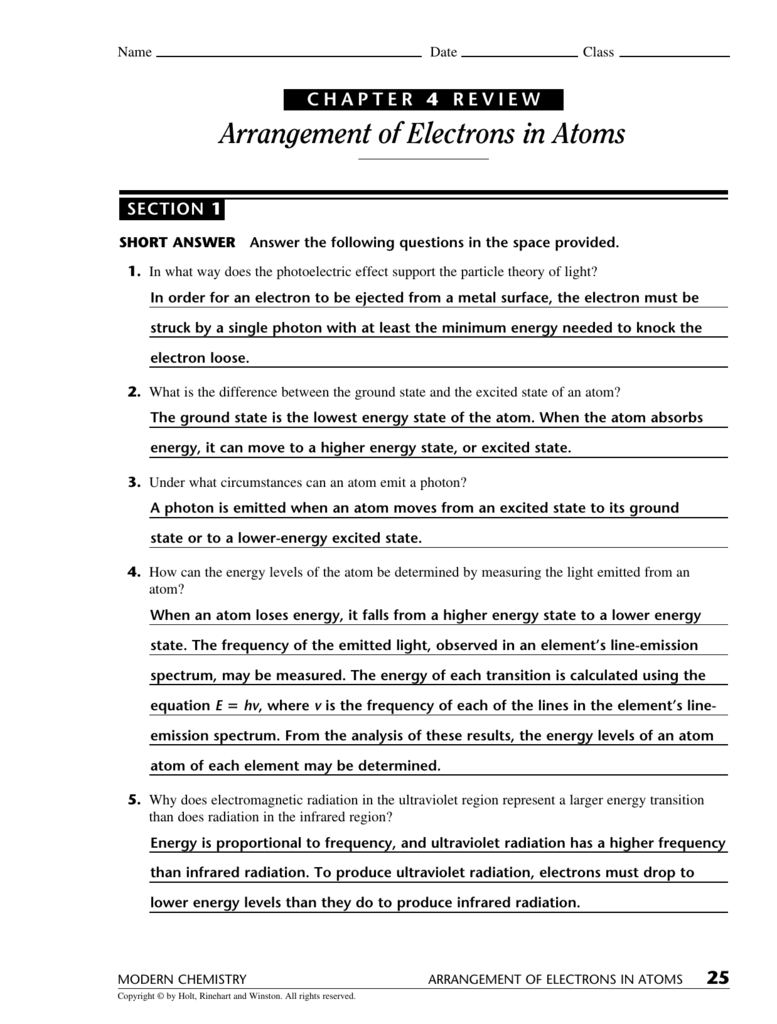
Arrangement of electrons in atoms the following pages contain the bulk (but not all) of the information for the chapter 4 test. The energy of each transition is calculated using the equation e = hv,. A form of energy that exhibits wavelike behavior as it. Web know how a line spectrum is produced. 1s 2 2s 2 2p 2;
PPT Chapter 4 Arrangement of Electrons in Atoms PowerPoint
Know how to calculate number of electrons. Web study flashcards on chapter 4 test review: Quickly memorize the terms, phrases and much more. Web higher energy state to a lower energy state. Focus on this content, but make sure to review class notes,.
Arrangement of Electrons in Atoms
Web this is a quick review of all the last parts of my honors chemistry notes on chapter 4. D) a position where an electron cannot exist. _____ compared with an electron for which n = 2, an electron for which n = 4 has more (a) spin. Web study flashcards on chapter 4 test review: Know the definition of.
Arrangement of Electrons in Atoms
_____ compared with an electron for which n = 2, an electron for which n = 4 has more (a) spin. Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons. Arrangement of electron in atoms at cram.com. The energy of each transition is calculated using the equation e = hv,..
electron Definition, Mass, & Facts Britannica
Know what must happen for an electron to move between the ground and excited states. C) a position where an electron probably exists. *** c) a position where an electron probably. Web science physics chemistry chapter 4 arrangement of electrons in atoms review term 1 / 44 the product of the frequency and the wavelength of a wave equals the.
Arrangement of Electrons in Atoms The Development of a New Atomic Model
As an electron moves from n=1 to n=4 energy is being. *** c) a position where an electron probably. Explain the mathematical relationship among the speed, wavelength, and frequency of electromagnetic radiation. 1s 2 2s 2 2p 1; Web study flashcards on chapter 4 test review:
Electrons in Atoms
_____ compared with an electron for which n = 2, an electron for which n = 4 has more (a) spin. 1s 2 2s 2 2p 1; 1s 2 2s 2 2p 4… C) a position where an electron probably exists. Web chemistry arrangement of electrons in atoms chapter 4 test review.
Valence Electrons Simple Definition Ciencias, Politica, Religion A
Learn about atomic orbital, the four quantum numbers (principal, angular momentum, magnetic, and spin), and how to write quantum. C) a position where an electron probably exists. Quickly memorize the terms, phrases and much more. Focus on this content, but make sure to review class notes,. Click the card to flip 👆.
PPT Chapter 5 Arrangement of electrons in atoms PowerPoint
1s 2 2s 2 2p 1; Learn about atomic orbital, the four quantum numbers (principal, angular momentum, magnetic, and spin), and how to write quantum. As an electron moves from n=1 to n=4 energy is being. Know how to calculate number of electrons. Web science physics chemistry chapter 4 arrangement of electrons in atoms review term 1 / 44 the.
1S 2 2S 2 2P 4…
1s 2 2s 2 2p 3; Quickly memorize the terms, phrases and much more. _____ according to bohr, which is the point in the figure below where electrons. C) a position where an electron probably exists.
_____ How Many Electrons Can An Energy Level Of N = 2 Hold?
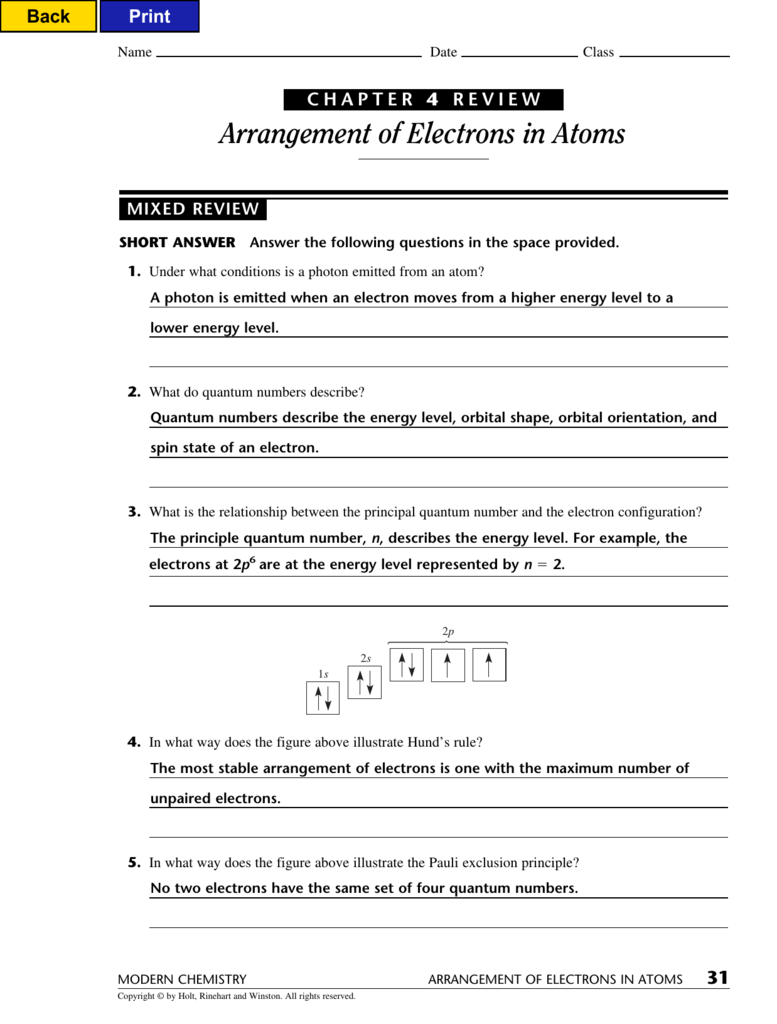
Click the card to flip 👆. Click the card to flip 👆. Electrons exist only in very specific energy states for atoms. Thus, the electron configurations for the next six atoms are as follows:
A Form Of Energy That Exhibits Wavelike Behavior As It.
(a) 32 (c) 8 (b) 24 (d) 6 5. _____ according to bohr, which is the point in the figure below where electrons. Web know how a line spectrum is produced. Web quantum numbers describe specific properties of an electron.
Explain The Mathematical Relationship Among The Speed, Wavelength, And Frequency Of Electromagnetic Radiation.
Learn about atomic orbital, the four quantum numbers (principal, angular momentum, magnetic, and spin), and how to write quantum. Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Web chemistry arrangement of electrons in atoms chapter 4 test review. Arrangement of electrons in atoms the following pages contain the bulk (but not all) of the information for the chapter 4 test.