Can Hf Form Hydrogen Bonds
Can Hf Form Hydrogen Bonds - The other lone pairs are essentially. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. [17] [18] typical enthalpies in vapor include: Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs. But in gas phase , molecules are far apart. The total number of hydrogen. Each water molecule forms 4 hydrogen bonding. According to my information, hydrogen bonding present in ice. Such a bond is weaker than an ionic bond or.
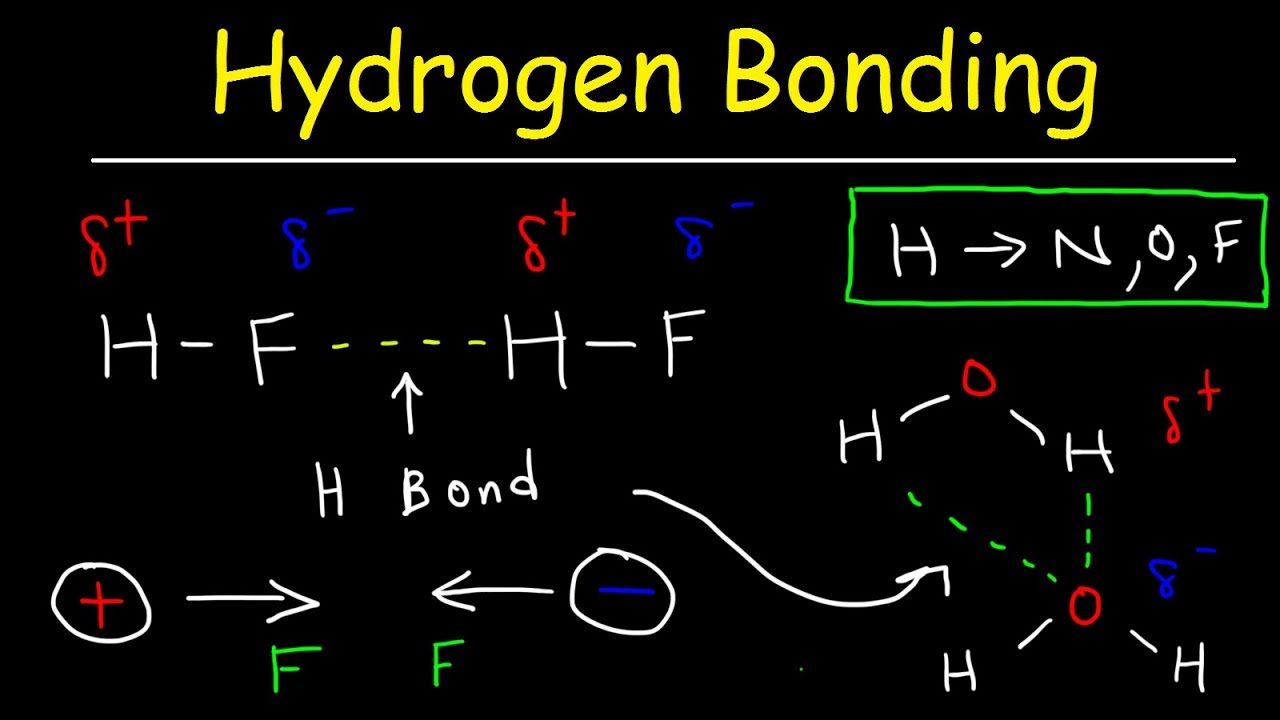
Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules. Web answer (1 of 3): Such a bond is weaker than an ionic bond or. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; [17] [18] typical enthalpies in vapor include: Web as a liquid, hf forms relatively strong hydrogen bonds, hence its relatively high boiling point. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Each water molecule forms 4 hydrogen bonding. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs.
Web hydrofluoric acid (hf): Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Each water molecule forms 4 hydrogen bonding. Molecules with hydrogen bonds will. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules. Such a bond is weaker than an ionic bond or. The total number of hydrogen. Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond.
Properties of Water Presentation Biology
But in gas phase , molecules are far apart. The total number of hydrogen. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. [17] [18] typical enthalpies in vapor include: Web hydrogen bonding, interaction involving a hydrogen.
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
According to my information, hydrogen bonding present in ice. The total number of hydrogen. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Molecules with hydrogen bonds will. Web hydrogen bonds also occur when hydrogen is bonded.
Why do H2O Molecules form more Hydrogen Bonds compared to NH3 and HF
According to my information, hydrogen bonding present in ice. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. But in gas phase , molecules are far apart. Each water molecule forms 4 hydrogen bonding. Web hydrogen bonding,.
chemistry Intermolecular Hydrogen Bonding
[17] [18] typical enthalpies in vapor include: Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Each water molecule forms 4 hydrogen bonding. The total number of hydrogen. Web hydrogen bonding, interaction involving a hydrogen atom located.
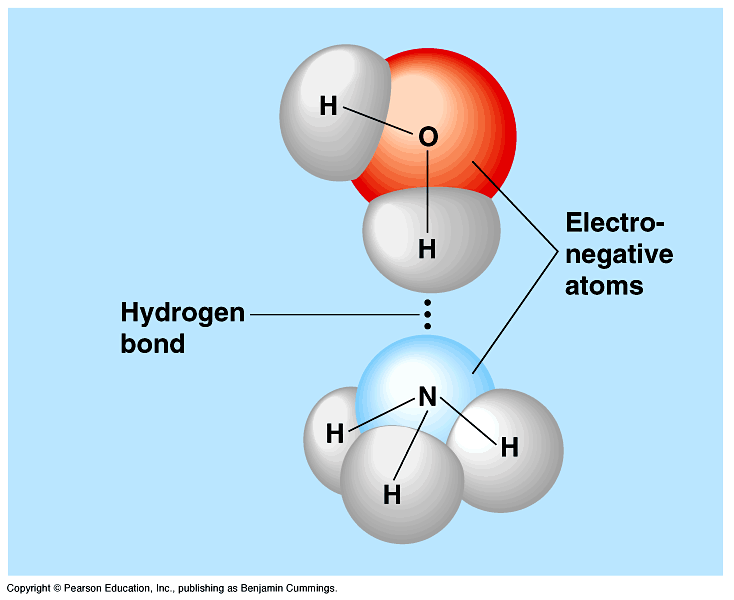
Diagram Of Water Molecules Hydrogen Bonding
The total number of hydrogen. Molecules with hydrogen bonds will. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: [17] [18] typical enthalpies in vapor include: Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules.
How can the hydrogen bonds in solid HF be best represented? ECHEMI
Molecules with hydrogen bonds will. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Such a bond is weaker than an ionic bond or. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. According to my information, hydrogen bonding.
Hydrogen A Hydrogen Bond Is
Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Each water molecule forms 4 hydrogen bonding. The total number of hydrogen. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen.
Bonds That Hold Water Molecules Together / Intermolecular Forces
According to my information, hydrogen bonding present in ice. But in gas phase , molecules are far apart. The total number of hydrogen. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Such a bond is weaker than an ionic bond or.
chemical bonding hydrogen bonding notes for neet and jee
Web answer (1 of 3): Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: The other lone pairs are essentially. Web.
How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding
Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. The hf molecules, with a. Molecules with hydrogen bonds will. Web as a liquid, hf forms relatively strong hydrogen bonds, hence its relatively high boiling point. Hydrofluoric acid.
The Hf Molecules, With A.
But in gas phase , molecules are far apart. [17] [18] typical enthalpies in vapor include: The other lone pairs are essentially. Molecules with hydrogen bonds will.
Such A Bond Is Weaker Than An Ionic Bond Or.
Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Each water molecule forms 4 hydrogen bonding.
Web Hydrogen Bonding, Interaction Involving A Hydrogen Atom Located Between A Pair Of Other Atoms Having A High Affinity For Electrons;
Web in hf each molecule has one hydrogen atom which can form a hydrogen bond, and there are three lone pairs of electrons on the fluorine atom. Web as a liquid, hf forms relatively strong hydrogen bonds, hence its relatively high boiling point. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs.
The Total Number Of Hydrogen.
Web hydrofluoric acid (hf): Web answer (1 of 3): Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules. According to my information, hydrogen bonding present in ice.
.PNG)