Chapter 2 Chemistry Of Life Answer Key
Chapter 2 Chemistry Of Life Answer Key - One particular type of atom which cannot be broken down into simpler substances. Contain hydrogen, oxygen, nitrogen, phosphorus,. The center of the atom that is formed by protons and neutrons bound together with strong forces. Consistent, unifying themes in each chapter such as the big picture and cycle of life sections tie your. In this model, the atoms are carbon (black), oxygen (red),. Web key, illustrates the most current scientific knowledge and makes the information more accessible. Web the first section of the chapter 2 answer key focuses on the building blocks of life. Web the main source of energy for living things. The answer key provides clear explanations of these. Because they have the same # of electrons what are the main types of chemical.
Substance made of atoms of different. Web 1 / 85 flashcards created by dillypapa chapter 2 the chemistry of life study guide terms in this set (85) the main source of energy for living things carbohydrates help carry out chemical reactions proteins. Important parts of biological membranes. Help carry out chemical reactions. Answer key 1 chapter 2 chemistry of life test answer key. The center of the atom that is formed by protons and neutrons bound together with strong forces. Web the first section of the chapter 2 answer key focuses on the building blocks of life. Web key, illustrates the most current scientific knowledge and makes the information more accessible. How do chemicals combine and break apart inside living things? Why are the properties of water important to organisms?
2 o, nacl, oh, no 3, po. In this model, the atoms are carbon (black), oxygen (red),. 4 the physical and chemical properties of a compound are usually very different from those of the elements from which it is formed. Why are the properties of water important to organisms? How do organisms use different types of carbon compounds? Important parts of biological membranes. Protons neutrons electrons why do isotopes of the same element have the same chemical properties? Polarity in a water molecule is caused by. Web an atom or molecule that has gained or lost one or more electrons. The basic unit of matter.
Biology Chapter 2 The Chemistry Of Life Study Guide Study Poster
The answer key provides clear explanations of these. Answer key 1 chapter 2 chemistry of life test answer key. Why are the properties of water important to organisms? A 6 chapter 2 chemistry of life test answer key. A base is a compound that can form a basic solution when dissolved.
Unit 5 Test Review Biology Answer Key / Cells The Units Of Life
4 the physical and chemical properties of a compound are usually very different from those of the elements from which it is formed. Help carry out chemical reactions. Why are the properties of water important to organisms? Answer key 1 chapter 2 chemistry of life test answer key. This chapter provides the chemistry background needed to understand the human body,.
chapter 2 The chemistry of life Crossword WordMint
Web chapter 2 the chemistry of life concept map answer key | metro map. What is the matter in organisms made of? The answer key provides clear explanations of these. Figure 2.1 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key.
Biology Chapter 2 The Chemistry Of Life Worksheet Answers —
Figure 2.1 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. Why are the properties of water important to organisms? Web learn test match created by blakebahos32 terms in this set (72) what are the subatomic particles that make up an atom? How do organisms use different types of carbon compounds? We next provide the overview.
Chapter 2 Chemical Basis Of Life Study Guide Answers Study Poster
Important parts of biological membranes. A variant form of an atom. Web an atom or molecule that has gained or lost one or more electrons. A 6 chapter 2 chemistry of life test answer key. The center of the atom that is formed by protons and neutrons bound together with strong forces.
12th class Solution Chapter 2 Chemistry handwritten notes pdf here by
Web chapter 2 the chemistry of life concept map answer key | metro map. Contain hydrogen, oxygen, nitrogen, phosphorus,. Figure 2.1 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. Consistent, unifying themes in each chapter such as the big picture and cycle of life sections tie your. The amount of energy associated with the movement.
Chemistry of Life Study Guide Answer Key Ion Chemical Elements
A variant form of an atom. We next provide the overview of silicon chemistry section 3. Protons neutrons electrons why do isotopes of the same element have the same chemical properties? Web chapter 2 big idea: Web the first section of the chapter 2 answer key focuses on the building blocks of life.
PPT Chapter 2 Chemistry of Life PowerPoint Presentation, free
The answer key provides clear explanations of these. One particular type of atom which cannot be broken down into simpler substances. Why are the properties of water important to organisms? Web the subatomic particles that make up atoms are protons, neutrons, and electrons. Contain hydrogen, oxygen, nitrogen, phosphorus,.
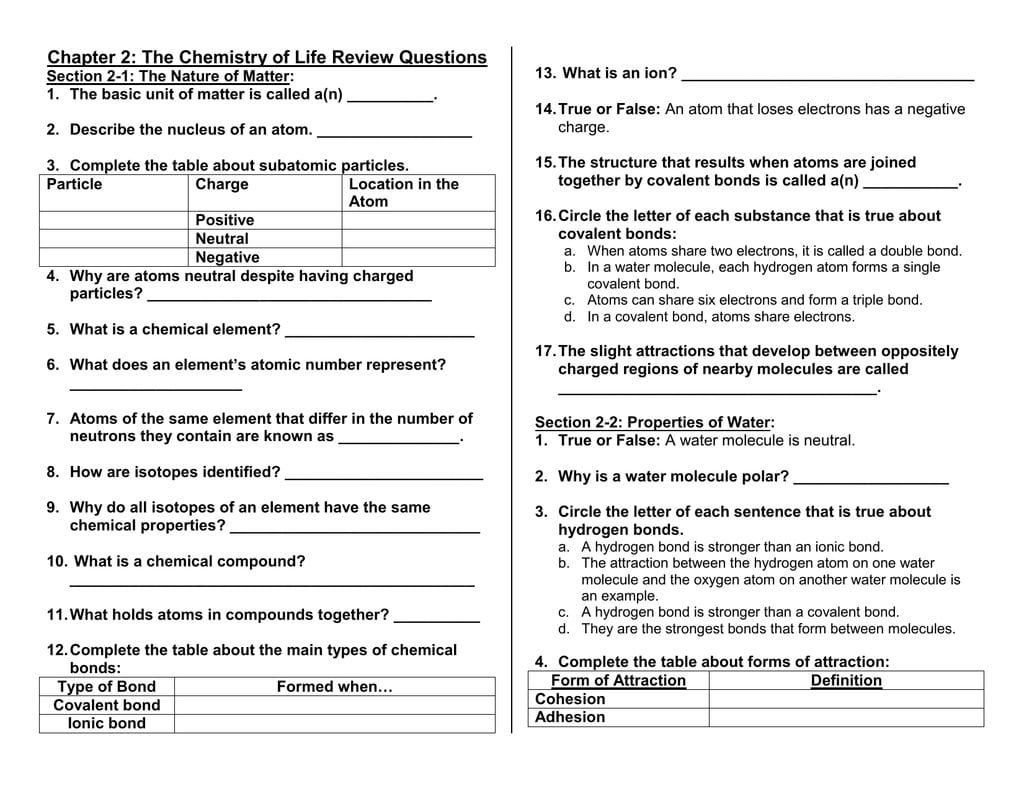
Chapter 2 The Chemistry Of Life Review Questions —
Protons neutrons electrons why do isotopes of the same element have the same chemical properties? In this model, the atoms are carbon (black), oxygen (red),. Web chapter 2 big idea: The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key. 2 o, nacl, oh, no 3, po.
chapter 2 The chemistry of life Crossword WordMint
A base is a compound that can form a basic solution when dissolved. The answer key provides clear explanations of these. The chapter describes biochemical compounds and reactions as well as the significance of water to life. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key. Web the subatomic particles that make up atoms are protons, neutrons,.
This Is Due To Their Greater Concentration Of H Ions Than Pure Water.
One particular type of atom which cannot be broken down into simpler substances. Web chapter 2 the chemistry of life concept map answer key | metro map. 4 the physical and chemical properties of a compound are usually very different from those of the elements from which it is formed. Help carry out chemical reactions.
Web The First Section Of The Chapter 2 Answer Key Focuses On The Building Blocks Of Life.
Contain hydrogen, oxygen, nitrogen, phosphorus,. The basic unit of matter. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key. In this model, the atoms are carbon (black), oxygen (red),.
The Chapter Describes Biochemical Compounds And Reactions As Well As The Significance Of Water To Life.
Matter and energy 2.1 gq: This dna molecules, like all molecules, is made up of atoms joined together in bonds. Web learn test match created by abtastic2020 terms in this set (83) the three particles that make up an atom are a) protons, neutrons, and isotopes b) neutrons, isotopes and electrons c) positive, negatives, and. Introduction to the chemistry of life.
This Chapter Provides The Chemistry Background Needed To Understand The Human Body, Its Functions, And Its Processes.
We next provide the overview of silicon chemistry section 3. Because they have the same # of electrons what are the main types of chemical. Web substance formed by the chemical combination of 2 or more elements. The amount of energy associated with the movement of the atoms and molecules in a body of matter.