How Many Hydrogen Bonds Can One Water Molecule Form
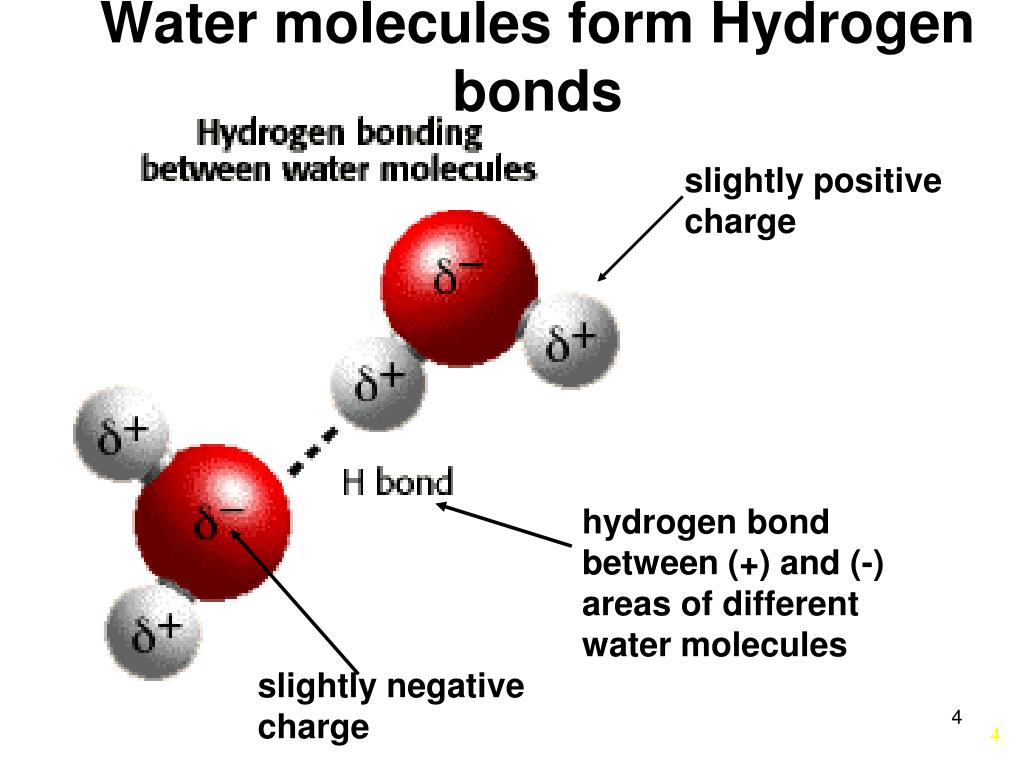
How Many Hydrogen Bonds Can One Water Molecule Form - The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. It can donate two hydrogen atoms and can accept two. That means that every hydrogen will. Web consequently, they can form hydrogen bonds with water. Each water molecule is surrounded by four neighboring h 2 os. Web each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water. On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one. Web notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Both an oxygen atom and 2 hydrogen atoms in one molecule can.
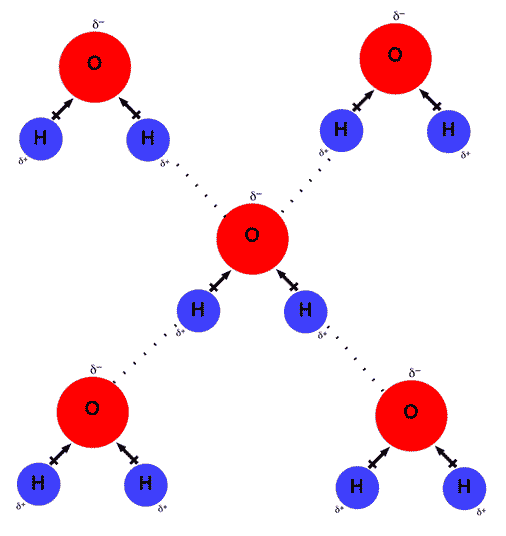
Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Web a single water molecule can usually make three hydrogen bonds but in some cases it can make up to four. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web consequently, they can form hydrogen bonds with water. There are exactly the right numbers of + hydrogens. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Two with the hydrogen atoms and two with the with the. A one b two c three d four medium solution verified by toppr correct option is d) water is. Web notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one.
Two with the hydrogen atoms and two with the with the. It can donate two hydrogen atoms and can accept two. The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water. Each water molecule is surrounded by four neighboring h 2 os. There are exactly the right numbers of + hydrogens. Two with the hydrogen atoms and two with the with the. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one. Web in hydrogen fluoride, the problem is a shortage of hydrogens. Web notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules:
PPT Water Chemistry & Properties of Water PowerPoint Presentation
Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Two with the hydrogen atoms and two with the with the. That means that every hydrogen will. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. There are.
Water Polar Covalent Bond My XXX Hot Girl
That means that every hydrogen will. Two with the hydrogen atoms and two with the with the. The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water. A one b two c three d four medium solution verified by toppr correct option is d) water is. There are exactly the right numbers of + hydrogens.
Water Molecule Model Building Activity 教育
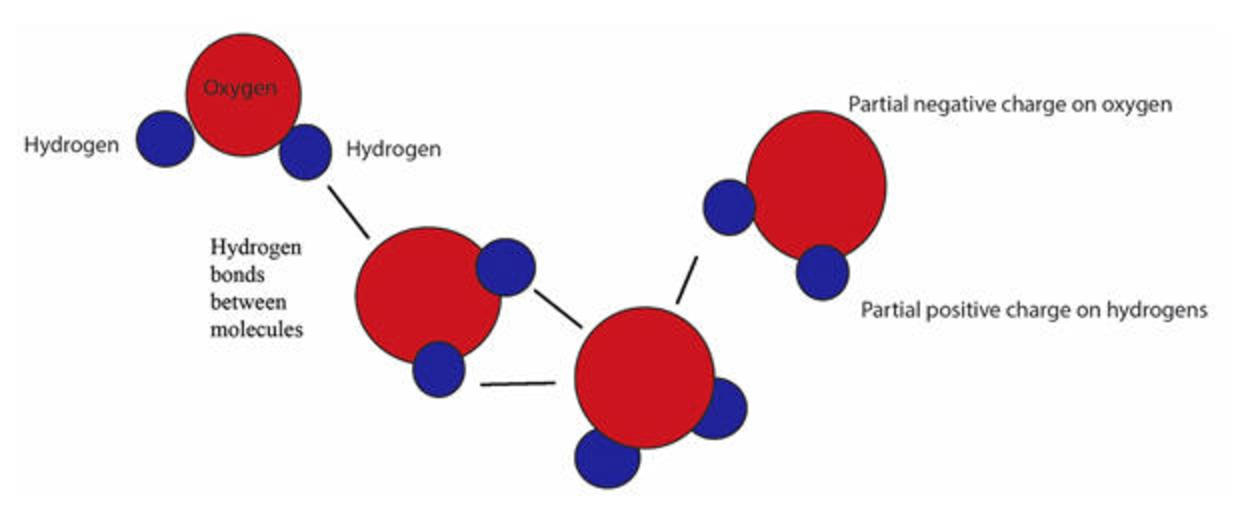
Web each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water. That means that every hydrogen will. Each water molecule is surrounded by four neighboring h 2 os. The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water. Web but.
The Unique Properties Of Water How Hydrogen Bonding Affects Our Body
Web a single water molecule can usually make three hydrogen bonds but in some cases it can make up to four. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. A one b two c three d four medium solution.
Water
Web each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water. Web the maximum possible number of hydrogen bonds formed by one water molecule is: Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms.
Nature up close Water molecules CBS News
Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. If your high school biology teacher is asking you this. Web each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen.
😎 What properties of water make it essential to life. Why Water Is
The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water. Web in hydrogen fluoride, the problem is a shortage of hydrogens. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. There are exactly the right numbers of + hydrogens. It can donate two hydrogen atoms and can accept.
Learn for free about math, art, computer programming, economics
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Two with the hydrogen atoms and two with the with the. A one b two.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Two with the hydrogen atoms and two with the with the. A one b two c three d four medium solution verified by toppr correct option is d) water is. If your high school biology teacher.
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Web in hydrogen fluoride, the problem is a shortage of hydrogens. Both an oxygen atom and 2 hydrogen atoms in one molecule can. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a.
Web Consequently, They Can Form Hydrogen Bonds With Water.
Two with the hydrogen atoms and two with the with the. Two with the hydrogen atoms and two with the with the. A one b two c three d four medium solution verified by toppr correct option is d) water is. 100% (2 ratings) a) one water molecule can form maximum of 4 hydrogen bonds with other water molecules.
There Are Exactly The Right Numbers Of + Hydrogens.
That means that every hydrogen will. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. It can donate two hydrogen atoms and can accept two. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure.
Each Water Molecule Is Surrounded By Four Neighboring H 2 Os.
On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one. Web in hydrogen fluoride, the problem is a shortage of hydrogens. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Web the maximum possible number of hydrogen bonds formed by one water molecule is:
If Your High School Biology Teacher Is Asking You This.
Both an oxygen atom and 2 hydrogen atoms in one molecule can. The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water. Web each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: