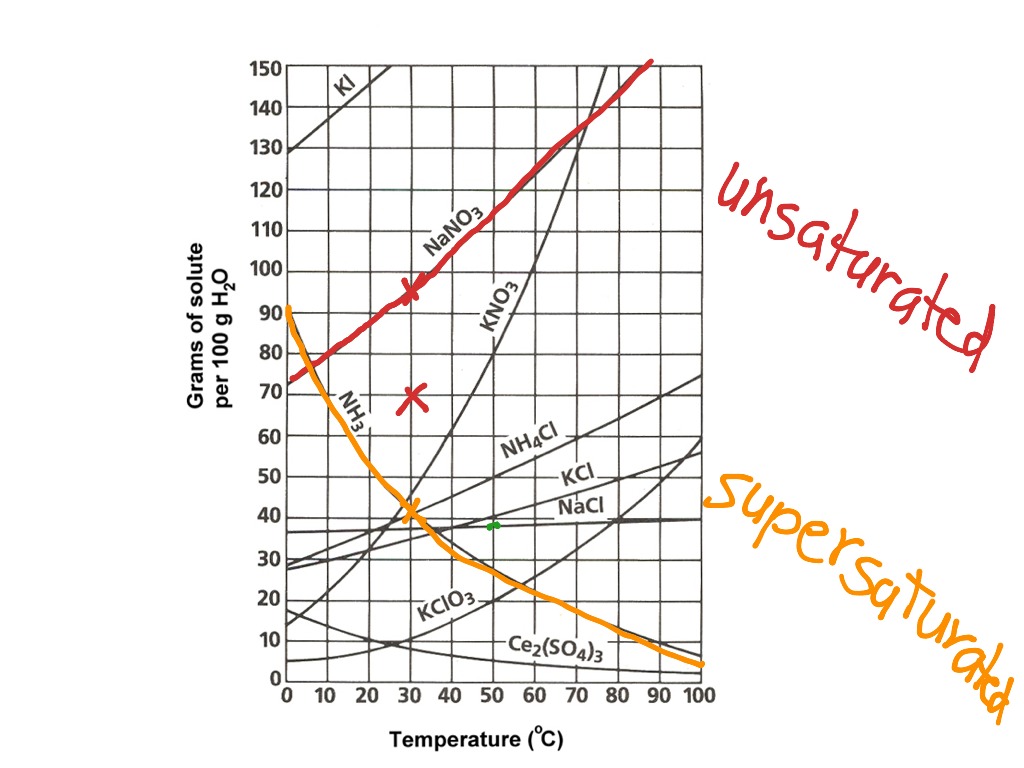
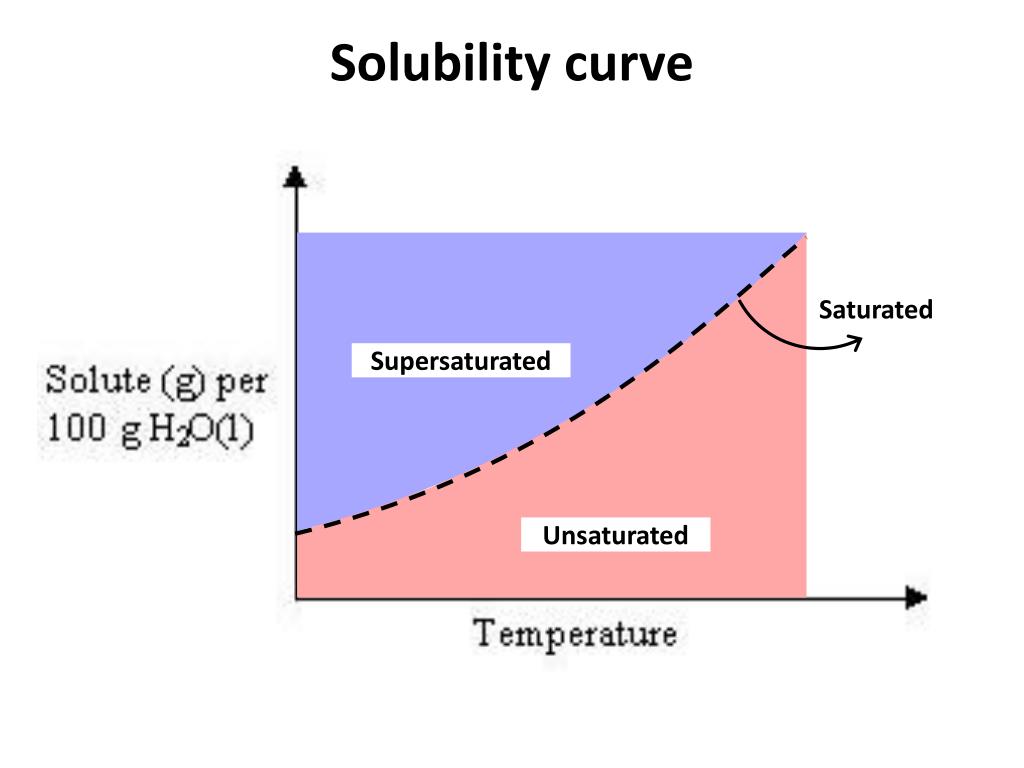
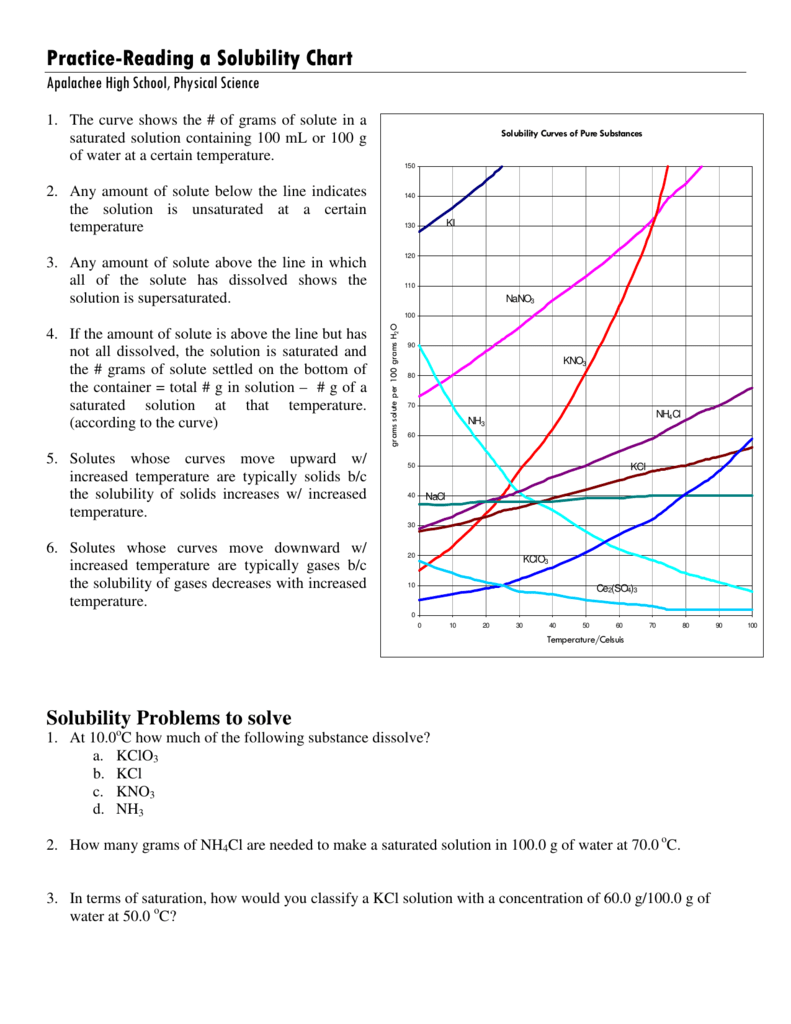
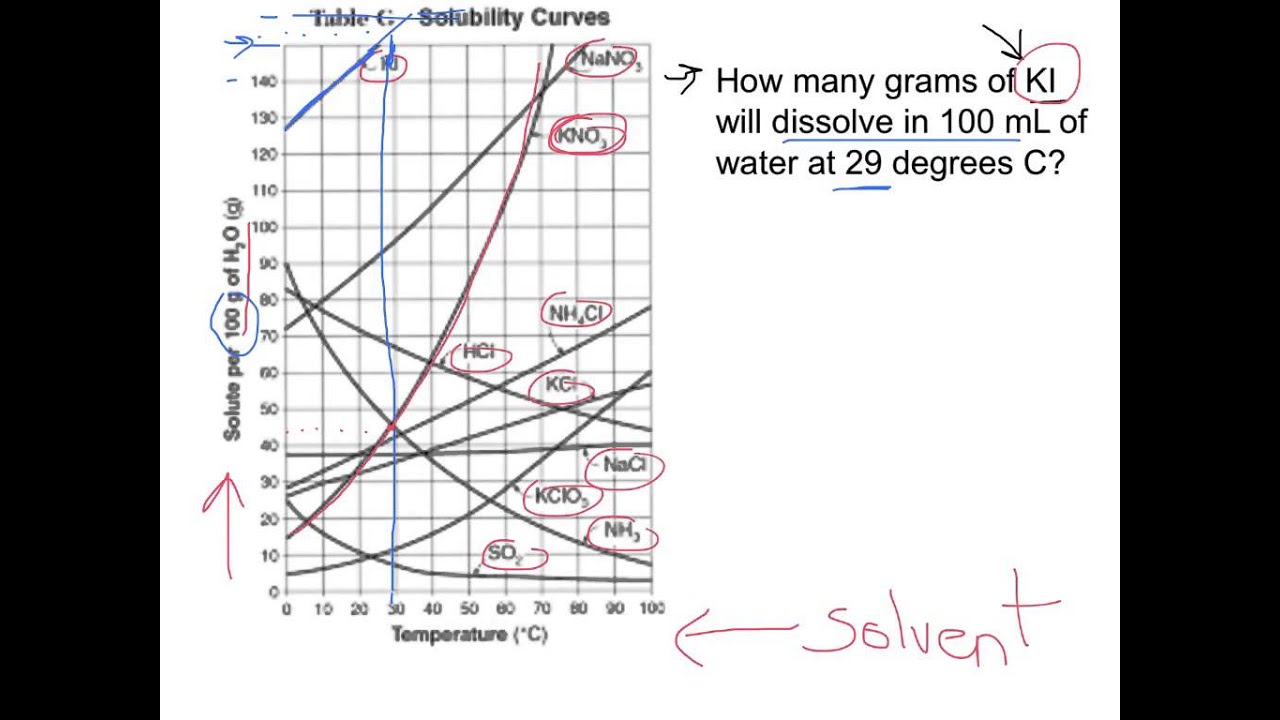
How To Read A Solubility Curve
How To Read A Solubility Curve - A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web how to read the solubility curve? The solubility curve line shows you with a saturated solution. Solubility curves for more than one substance are often drawn on the same. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution.
Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web how to read the solubility curve? The solubility curve line shows you with a saturated solution. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. Solubility curves for more than one substance are often drawn on the same. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c.
Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. The solubility curve line shows you with a saturated solution. Solubility curves for more than one substance are often drawn on the same. Web how to read the solubility curve? A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c.
Reading a SolubilityChart.doc Google Docs
Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. The solubility curve line shows you with a saturated solution. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount.
Read Solubility Curve Practice Answers / Solubility Graph Worksheet
Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. Saturated solution is basically the one with a full dissolved volume of solute in.
Solubility Curve Practice Problems Worksheet 1 Answers Chemistry
Web how to read the solubility curve? Solubility curves for more than one substance are often drawn on the same. The solubility curve line shows you with a saturated solution. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web since a solubility curve only shows you the amount of solute.
ShowMe solubility curve
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. The solubility curve line shows you with a saturated solution. Web how to read the solubility curve? Solubility curves for more than one substance are.
Reading Solubility Graphs YouTube
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less.
Read Solubility Curve Practice Answers Solubility Curve Worksheet
Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. The solubility curve line shows you with a saturated solution. Saturated solution is basically.
PPT Solubility curve PowerPoint Presentation, free download ID6497715
Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web how to read the solubility curve? Solubility curves for more than one substance are often drawn on the same. The solubility curve line shows.
PracticeReading a Solubility Chart
The solubility curve line shows you with a saturated solution. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. Saturated solution is basically.
Read Solubility Curve Practice Answers / Hw Solubility Curve Worksheet
The solubility curve line shows you with a saturated solution. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. A solubility curve is.
Reading solubility curves YouTube
Web how to read the solubility curve? The solubility curve line shows you with a saturated solution. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less.
The Solubility Curve Line Shows You With A Saturated Solution.
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web how to read the solubility curve?