Hydrogen And Oxygen React Chemically To Form Water
Hydrogen And Oxygen React Chemically To Form Water - For 2 moles hydrogen consumed, we need 1 mole of oxygen. How many grams of sulfur dioxide can be produced when 38.5 g of oxygen reacts? Yet, it is still common for reactions involving molecular compounds to be classified as redox reactions. Basically, you could see the former u.s. Web in a molecular compound, electrons are shared between atoms in a type of bond called a covalent bond. We need a stoichiometric equation for water synthesis: When you burn hydrogen and oxygen gas, the reaction is: Web hydrogen and oxygen react chemically to form water. How much water would form if 4.8 grams of hydrogen reacted with 38.4 grams of oxygen? Web when molecular hydrogen (h 2) and oxygen (o 2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water.
Basically, you could see the former u.s. But if the hydrogen atom no longer has its sole electron, is it still a hydrogen atom. 32 g of oxygen will react with 4 g of h2. Web the following equation, for example, does not guarantee that hydrogen will react with oxygen to form water. Write the balanced equation for the reaction. When hydrogen gas is reacted with oxygen gas, water is formed as the product. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web hydrogen + oxygen = water. Web when the gases hydrogen and oxygen are mixed and the mixture is ignited, water vapor forms (it will later condense to liquid water as the fire subsides). Web when molecular hydrogen (h 2) and oxygen (o 2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water.
Hydrogen is the reactant in excess, there will remain 5.155 moles of hydrogen. Web the following equation, for example, does not guarantee that hydrogen will react with oxygen to form water. Web when the gases dihydrogen sulfide and oxygen react, they form the gases sulfur dioxide and water vapor. Web hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web grant mason from the byu department of physics and astronomy demonstrates the reaction of hydrogen and oxygen to form water. 2hx2x(g) +ox2x(g) 2hx2ox(g) +energy 2 h x 2 x ( g) + o x 2 x ( g) 2 h x 2 o x ( g) + e n e r g y. Moles of dihydrogen = 4.8 ⋅ g 2.01 ⋅ g ⋅ mol−1 =. From the above equation, we can say that 4g of hydrogen will react with 32 g of oxygen. Web hydrogen and oxygen can form compounds other than diatomic gasses and water also.
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy
Over time, it will decompose with the following reaction: Web if 84.8 grams of hydrogen reacted with 54.8 grams of oxygen 61.65 grams of water will be formed. Mix the two gases together, add a spark or sufficient heat to provide the activation energy to start the reaction, and presto—instant water. The answer that mathew provided is correct, but i.
How to Make Oxygen and Hydrogen from Water Using Electrolysis
This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen: Web the following equation, for example, does not guarantee that hydrogen will react with oxygen to form water. H 2(g) +.
Learn how to do anything How to Make Oxygen and Hydrogen from Water
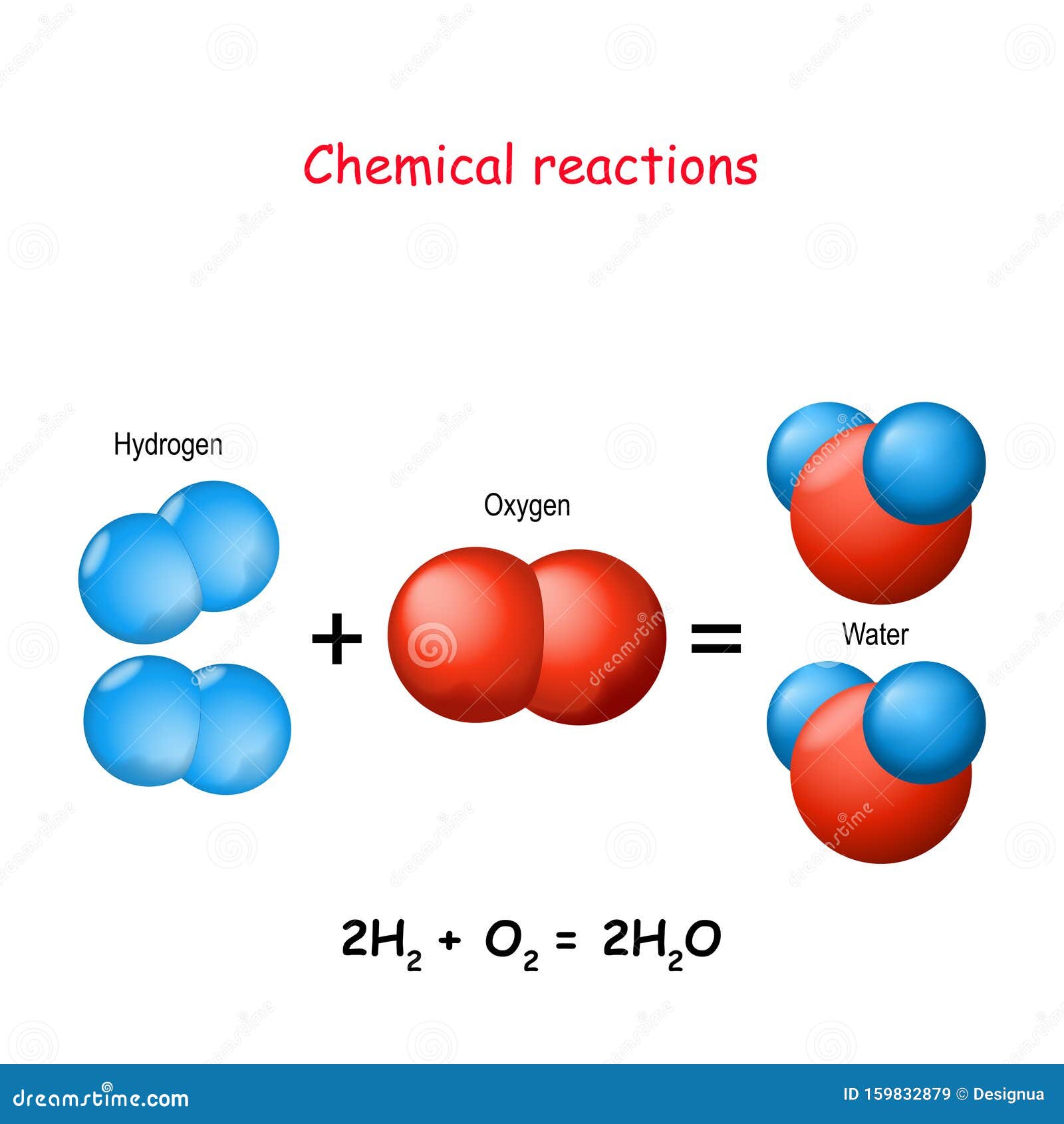
Web 2 h 2 + o 2 → 2 h 2 o how to make water in theory, it's easy to make water from hydrogen gas and oxygen gas. Web when molecular hydrogen (h 2) and oxygen (o 2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form.
Solved Hydrogen gas reacts with oxygen gas to produce water.
H 2(g) + 1 2o2(g) → h 2o(l) clearly, dihydrogen must be present in a 2:1 molar ratio with respect to dioxygen. Web hydrogenation (as shown in the figure below) is a chemical reaction that results from bonding hydrogen to organic compounds through the use of catalysts. Web chemistry chemical reactions chemical reactions and equations 1 answer anor277 sep 5,.
How to Make Oxygen and Hydrogen from Water Using Electrolysis
The reaction releases a lot of heat as you can see in this photo of the explosion of the hindenburg: From the above equation, we can say that 4g of hydrogen will react with 32 g of oxygen. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons..
Hydrogen and oxygen react chemically to form water. How much water
Web hydrogen + oxygen = water. Web the actual reaction to make water is a bit more complicated: For 2 moles hydrogen consumed, we need 1 mole of oxygen. Web hydrogen and oxygen are found as diatomic gaseous molecules of formula h x 2 \ce{h2} h x 2 and o x 2 \ce{o2} o x 2 , respectively. Water (.
Hydrogen and oxygen react chemically to form water. How much water
Mix the two gases together, add a spark or sufficient heat to provide the activation energy to start the reaction, and presto—instant water. Over time, it will decompose with the following reaction: Web 2 h 2 + o 2 → 2 h 2 o how to make water in theory, it's easy to make water from hydrogen gas and oxygen.
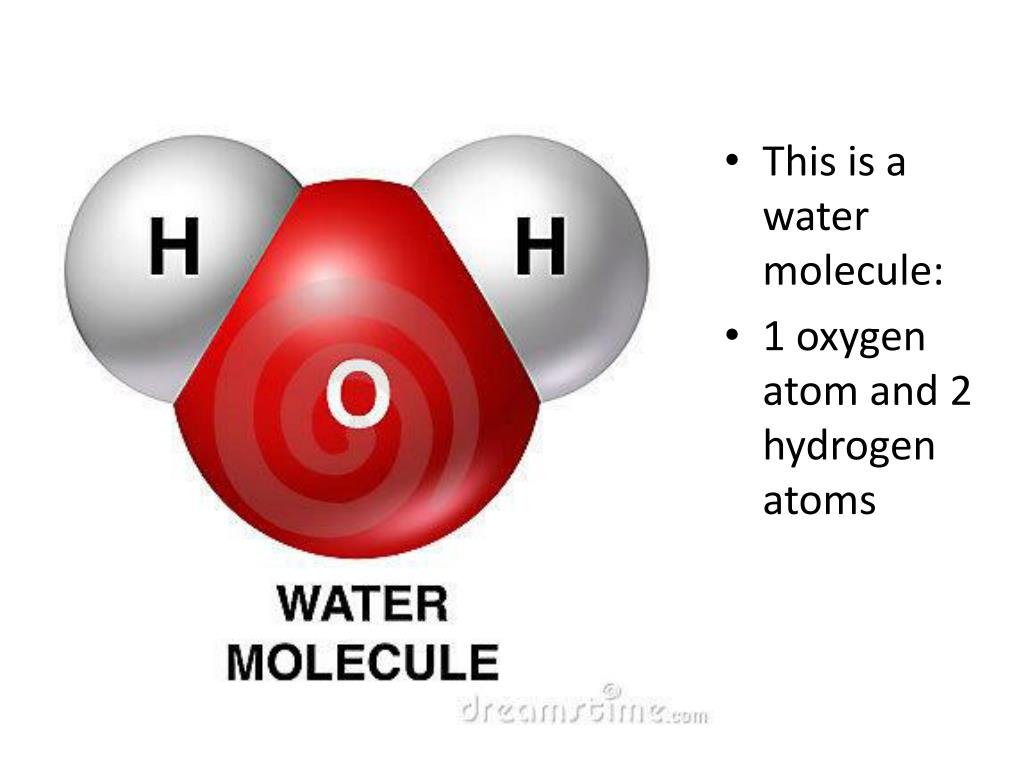
PPT This is a water molecule 1 oxygen atom and 2 hydrogen atoms
2h2 + o2 = 2h2o + energy. 2 h 2 ( g ) + o 2 ( g ) 2 h 2 o( g ) it is possible to fill a balloon with a mixture of hydrogen and oxygen and find that no reaction occurs until you touch the balloon with a flame. In this question, hydrogen is in excess.
SOLVEDWhen hydrogen is burned in oxygen to form water, the composition
The simple statement that water is made from hydrogen and oxygen doesn't give us a very clear picture of what really goes into the creation of a molecule of water. 2h 2 o 2 → 2h 2 o 2 + o 2 Hydrogen is the reactant in excess, there will remain 5.155 moles of hydrogen. 2h2 + o2 = 2h2o.
Compound Microscope Cartoon Vector 23417211
Mix the two gases together, add a spark or sufficient heat to provide the activation energy to start the reaction, and presto—instant water. In english, the equation says: A quick look at the chemical equation for the formation of water tells us more. Web hydrogen and oxygen react chemically to form water. H 2(g) + 1 2o2(g) → h 2o(l).
Space Shuttle As A Large Machine That Turns Hydrogen And Oxygen Into Water.
Mix the two gases together, add a spark or sufficient heat to provide the activation energy to start the reaction, and presto—instant water. How much water would form if 4.8 grams of hydrogen reacted with 38.4 grams of oxygen? The compound h 2 o 2, called hydrogen peroxide, is less stable than water, but can be synthesized nonetheless. Moles of oxygen = 34.8g / 32g/mole = 1.0875 moles.
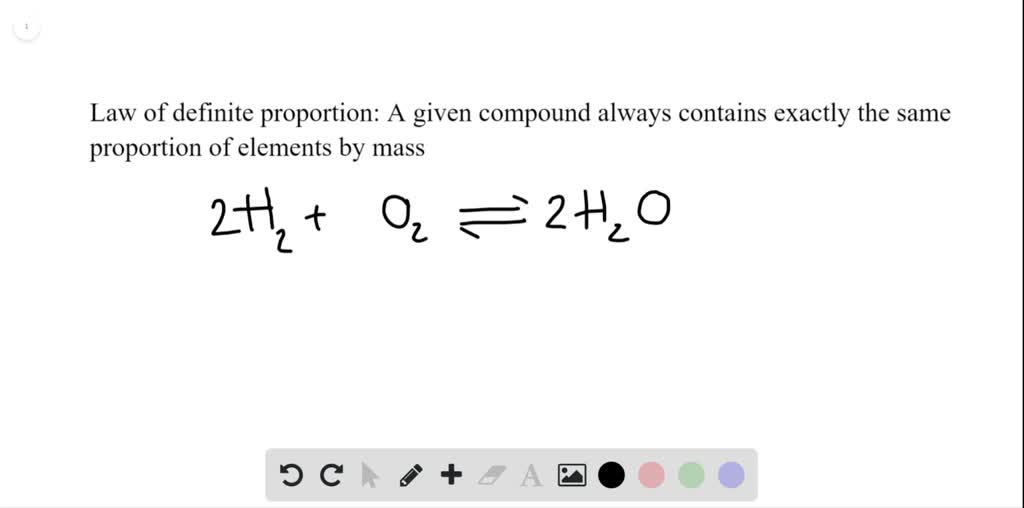
To Produce Two Molecules Of Water (H2O), Two Molecules Of Diatomic Hydrogen (H2) Must Be Combined With One Molecule Of Diatomic Oxygen (O2).
Web answer (1 of 7): Web if 84.8 grams of hydrogen reacted with 54.8 grams of oxygen 61.65 grams of water will be formed. 32 g of oxygen will react with 4 g of h2. Web the following equation, for example, does not guarantee that hydrogen will react with oxygen to form water.
In This Question, Hydrogen Is In Excess Amount.
In english, the equation says: This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Energy will be released in the process. Yet, it is still common for reactions involving molecular compounds to be classified as redox reactions.
Web Water Splitting Is The Chemical Reaction In Which Water Is Broken Down Into Oxygen And Hydrogen:
Web indeed, it's called combustion! Web in a molecular compound, electrons are shared between atoms in a type of bond called a covalent bond. When hydrogen gas is reacted with oxygen gas, water is formed as the product. For 2 moles hydrogen consumed, we need 1 mole of oxygen.