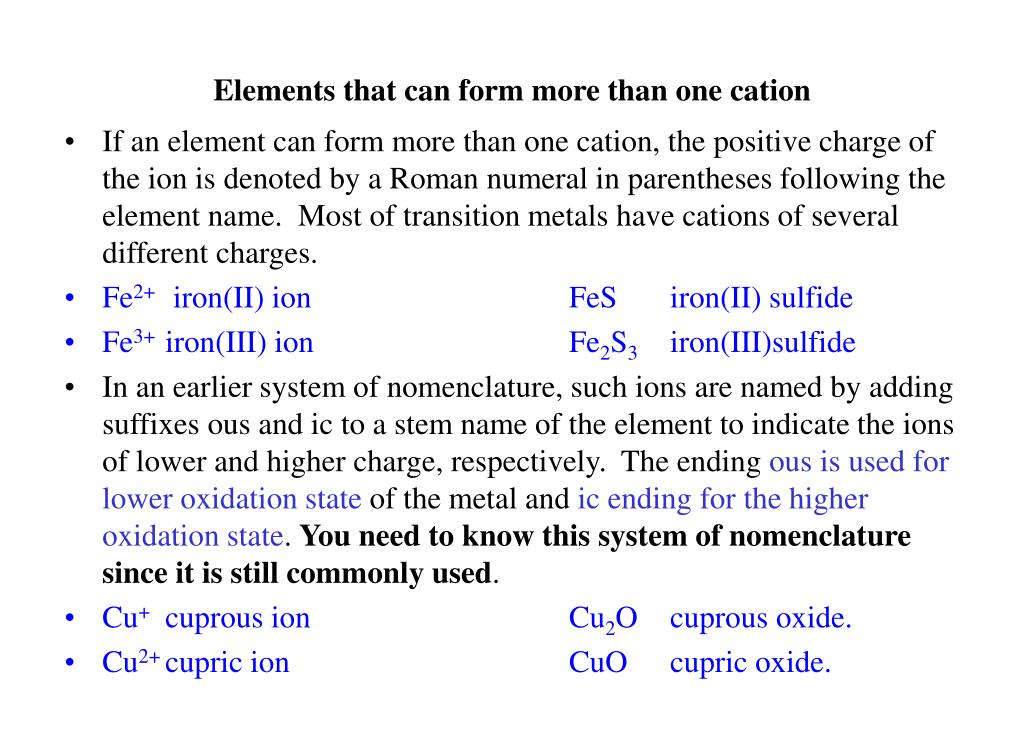
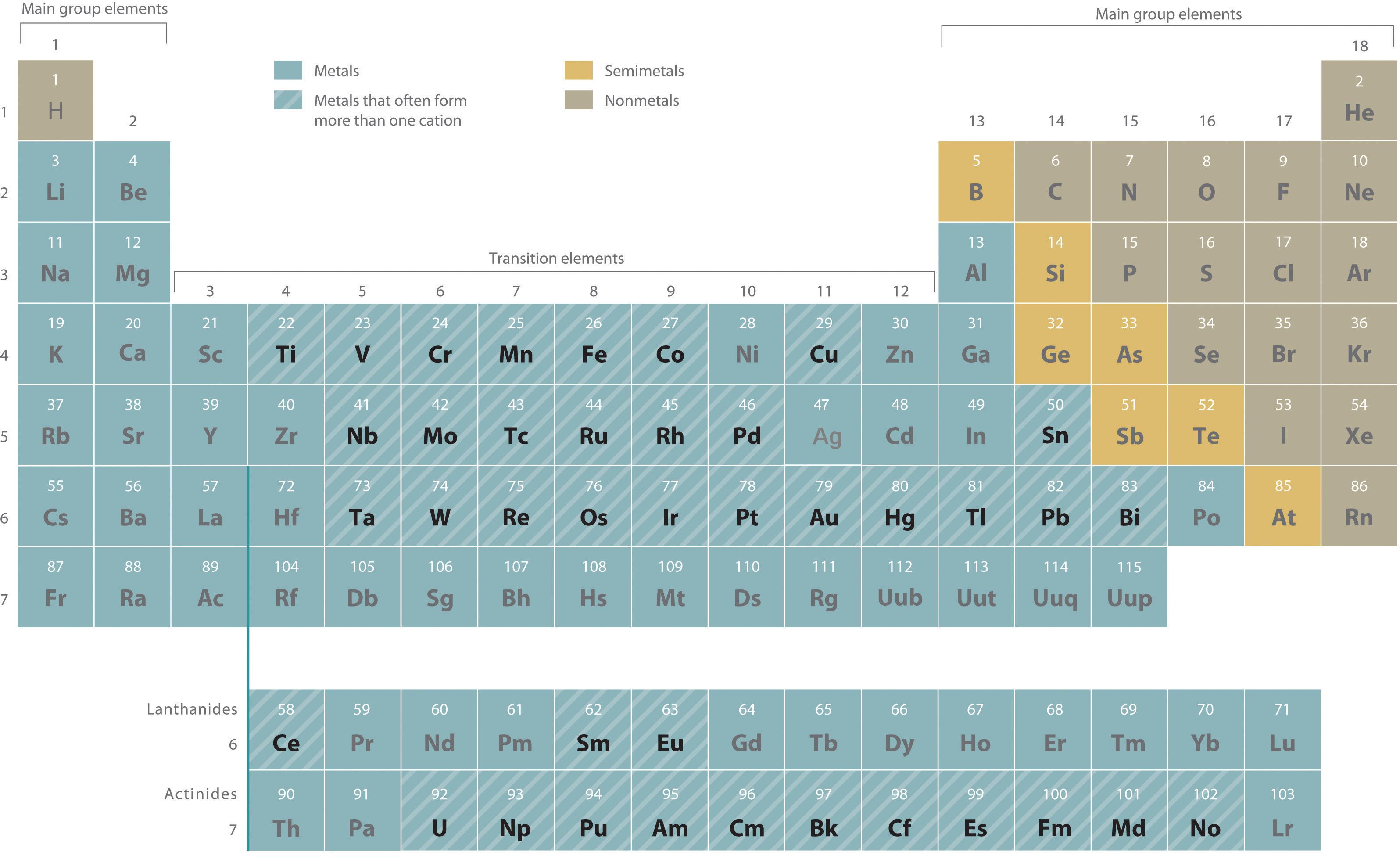
Identify Three Elements That Form More Than One Cation
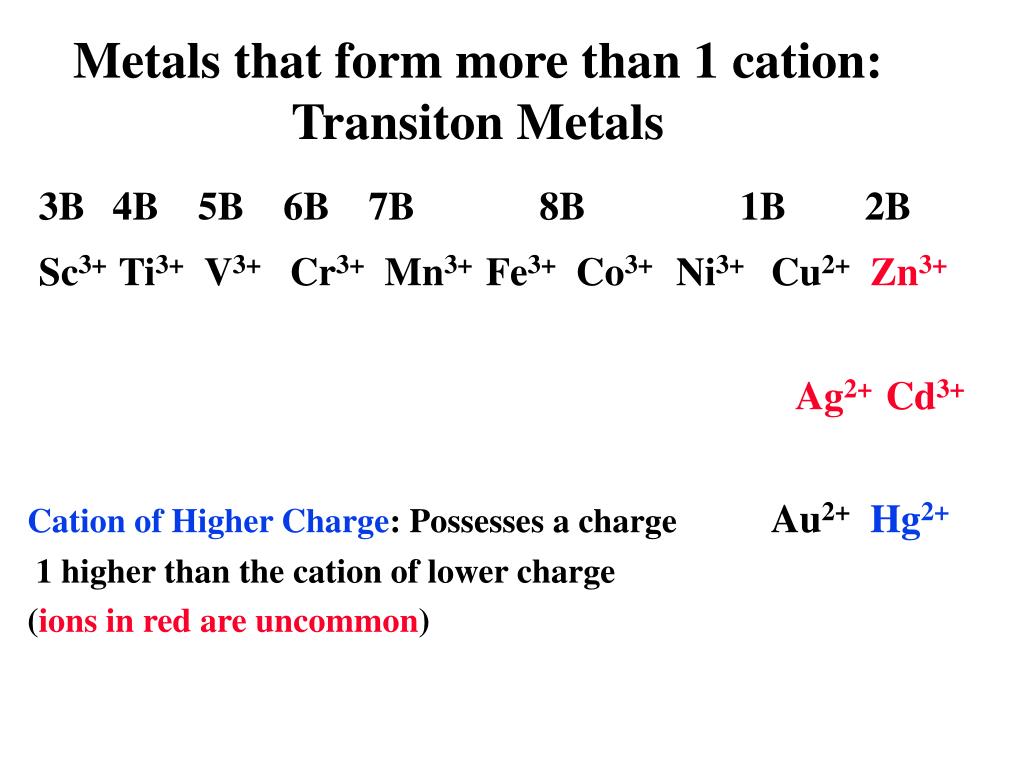
Identify Three Elements That Form More Than One Cation - Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Identify three elements that form only one cation. Web up to 24% cash back model 1: If an atom has lost one or more electrons, it is positively charged and is called a cation. Dec 5, 2016 any alkali metal or alkali earth metal will only. Based on the information in model 1: Cobalt is another element that can form. Ion charges for selected elements key questions 1. Chemistry matter net charge 1 answer dylan k. Web a b that cation is a transition metal that often forms find than one cation (table 2.5 common cations of metals that form more rather one ion).
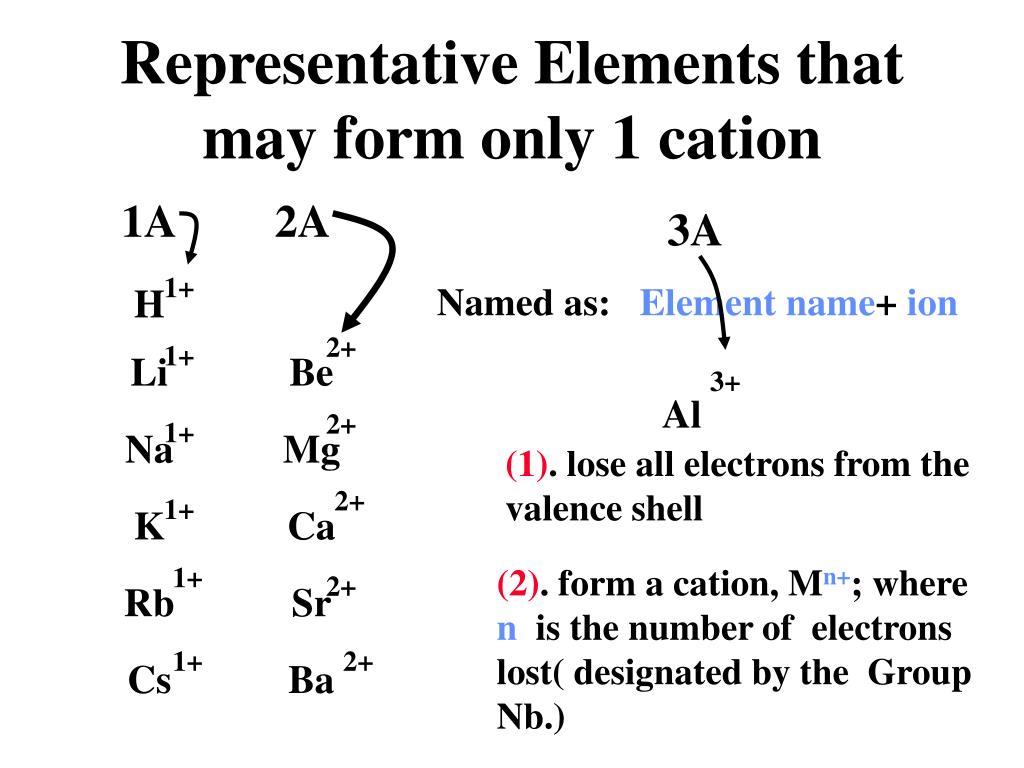
Identify three elements that form only one cation. Web hydrogen (h), lithium (li), sodium (na) identify three elements that form only one anion. Ielts® toefl® toeic® view all. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. The number of electrons lost, and so the. Dec 5, 2016 any alkali metal or alkali earth metal will only. Fluorine (f), chlorine (cl), bromine (br) identify three elements that form more than. Web what are three elements that form only one cation? Cobalt is another element that can form. Web a b that cation is a transition metal that often forms find than one cation (table 2.5 common cations of metals that form more rather one ion).
Web 12 rows naming binary ionic compounds with a metal that forms more than one type of cation. Web click here 👆 to get an answer to your question ️ identify three elements that form more than one cation The number of electrons lost, and so the. If an atom has lost one or more electrons, it is positively charged and is called a cation. Based on the information in model 1: Holding 100ml of water (ebkare)________________2. Web if an atom has gained one or more electrons, it is negatively charged and is called an anion. Web which element form cation? Fluorine (f), chlorine (cl), bromine (br) identify three elements that form more than. Ielts® toefl® toeic® view all.
PPT Naming Simple Compounds PowerPoint Presentation, free download
Easy solution verified by toppr any alkali metal or alkali earth metal will only form one cation. Fluorine (f), chlorine (cl), bromine (br) identify three elements that form more than. Wait a moment and try again. Holding 100ml of water (ebkare)________________2. Web what are three elements that form only one cation?
Three Elements That Form More Than One Cation Aulaiestpdm Blog
Web which element form cation? Web what are three elements that form only one cation? Web for a cation to form, one or more electrons must be lost, typically pulled away by atoms with a stronger affinity for them. The number of electrons lost, and so the. Wait a moment and try again.
PPT The Nomenclature of Binary Compounds PowerPoint Presentation ID
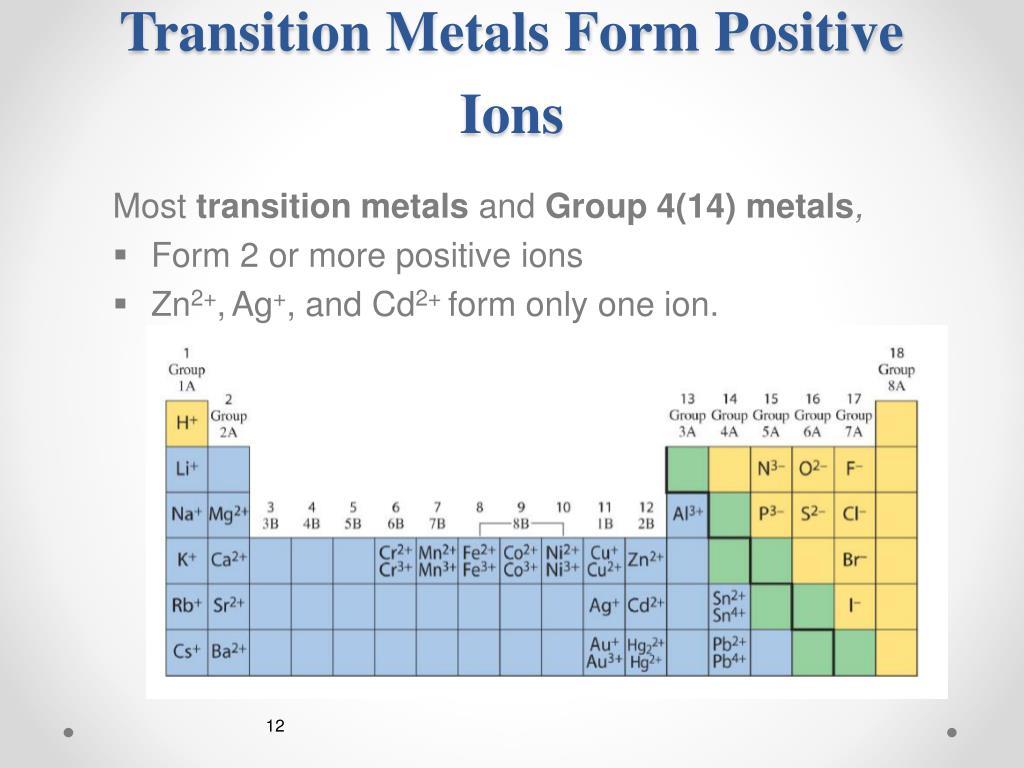
Web if an atom has gained one or more electrons, it is negatively charged and is called an anion. Web click here 👆 to get an answer to your question ️ identify three elements that form more than one cation For example, iron atoms can form 2+ cations or 3+ cations. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Holding 100ml.
PPT The Nomenclature of Binary Compounds PowerPoint Presentation ID
Web what are three elements that form only one cation? Holding 100ml of water (ebkare)________________2. Web what are three elements that form only one cation? Fluorine (f), chlorine (cl), bromine (br) identify three elements that form more than. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3.
Chapter 10.3 Naming Ionic Compounds Chemistry LibreTexts
Web which element form cation? Web click here 👆 to get an answer to your question ️ identify three elements that form more than one cation The number of electrons lost, and so the. Ielts® toefl® toeic® view all. For example, iron atoms can form 2+ cations or 3+ cations.
1 Name the ions formed by these elements and classify them as anions
Easy solution verified by toppr any alkali metal or alkali earth metal will only form one cation. Web click here 👆 to get an answer to your question ️ identify three elements that form more than one cation Web 12 rows naming binary ionic compounds with a metal that forms more than one type of cation. Web hydrogen (h), lithium.
Three Elements That Form More Than One Cation Aulaiestpdm Blog
Wait a moment and try again. Web click here 👆 to get an answer to your question ️ identify three elements that form more than one cation Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Web up to 24% cash back model 1: Web for a cation to form, one or more electrons must be lost, typically pulled away by atoms.
⚗️Identify three elements that form only one cation
Wait a moment and try again. Web hydrogen (h), lithium (li), sodium (na) identify three elements that form only one anion. Web uwec chem 103 stanley learn with flashcards, games, and more — for free. Identify three elements that form only one cation. Web which element form cation?
Image result for cations vs anions Chemistry classroom, Electron
Web if an atom has gained one or more electrons, it is negatively charged and is called an anion. Fluorine (f), chlorine (cl), bromine (br) identify three elements that form more than. Web hydrogen (h), lithium (li), sodium (na) identify three elements that form only one anion. Web a b that cation is a transition metal that often forms find.
Naming Ionic Compounds
Web if an atom has gained one or more electrons, it is negatively charged and is called an anion. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Chemistry matter net charge 1 answer dylan k. Web uwec chem 103 stanley learn with flashcards, games, and more — for free. For example, iron atoms can form 2+ cations or 3+ cations.
Web Click Here 👆 To Get An Answer To Your Question ️ Identify Three Elements That Form More Than One Cation
Fluorine (f), chlorine (cl), bromine (br) identify three elements that form more than. Based on the information in model 1: Web for a cation to form, one or more electrons must be lost, typically pulled away by atoms with a stronger affinity for them. Cobalt is another element that can form.
Identify Three Elements That Form Only One Cation.
Web if an atom has gained one or more electrons, it is negatively charged and is called an anion. Web hydrogen (h), lithium (li), sodium (na) identify three elements that form only one anion. Web 12 rows naming binary ionic compounds with a metal that forms more than one type of cation. Dec 5, 2016 any alkali metal or alkali earth metal will only.
Measuring 27 Ml Of Liquid (Daudgtear Ldnreiyc)________________3.
Web uwec chem 103 stanley learn with flashcards, games, and more — for free. Web a few elements, all metals, can form more than one possible charge. Wait a moment and try again. The number of electrons lost, and so the.
Web What Are Three Elements That Form Only One Cation?
If an atom has lost one or more electrons, it is positively charged and is called a cation. Holding 100ml of water (ebkare)________________2. Web what are three elements that form only one cation? Ion charges for selected elements key questions 1.