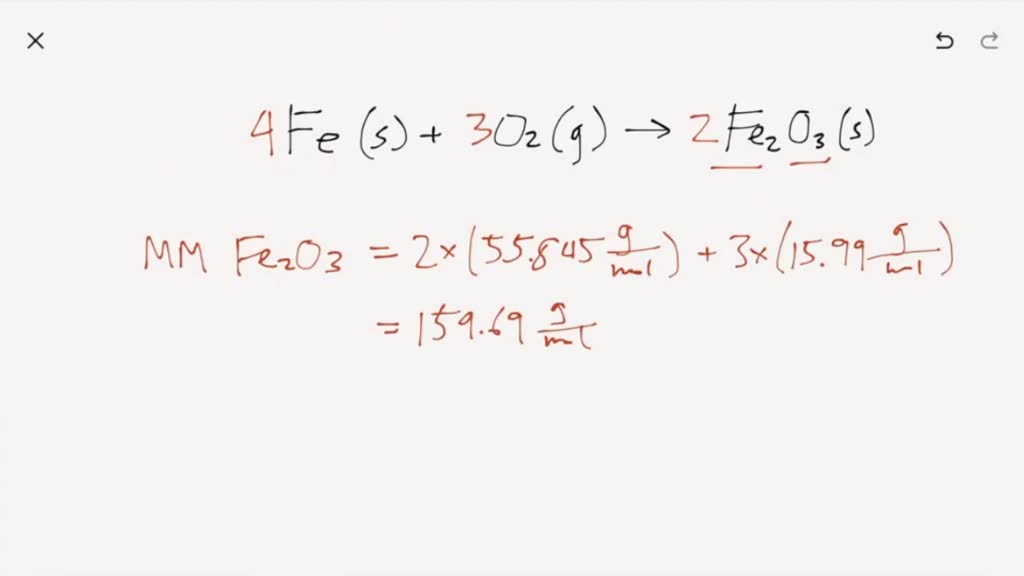Iron Reacts With Oxygen To Form Iron Iii Oxide
Iron Reacts With Oxygen To Form Iron Iii Oxide - Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Web iron reacts with oxygen to form iron (iii) oxide: Here is the word equation for the reaction: You know it is iron iii, not iron ii, because oxygen is always. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. 4fe (s)+3o2 (g) 2fe2o3 (s) suppose 13.0 g of iron (fe) is reacted with 19.7 g of oxygen. Iron reacts with oxygen at high temperatures to form iron (iii) oxide. Iron reacts with oxygen to form solid iron (iii) oxide. Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide.
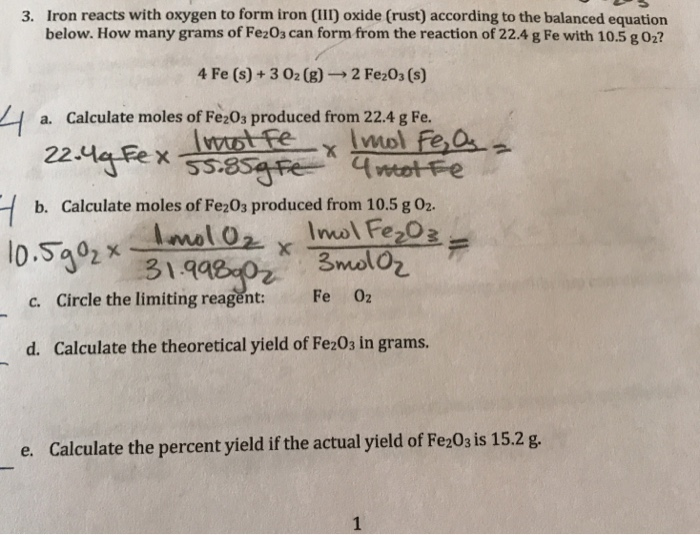
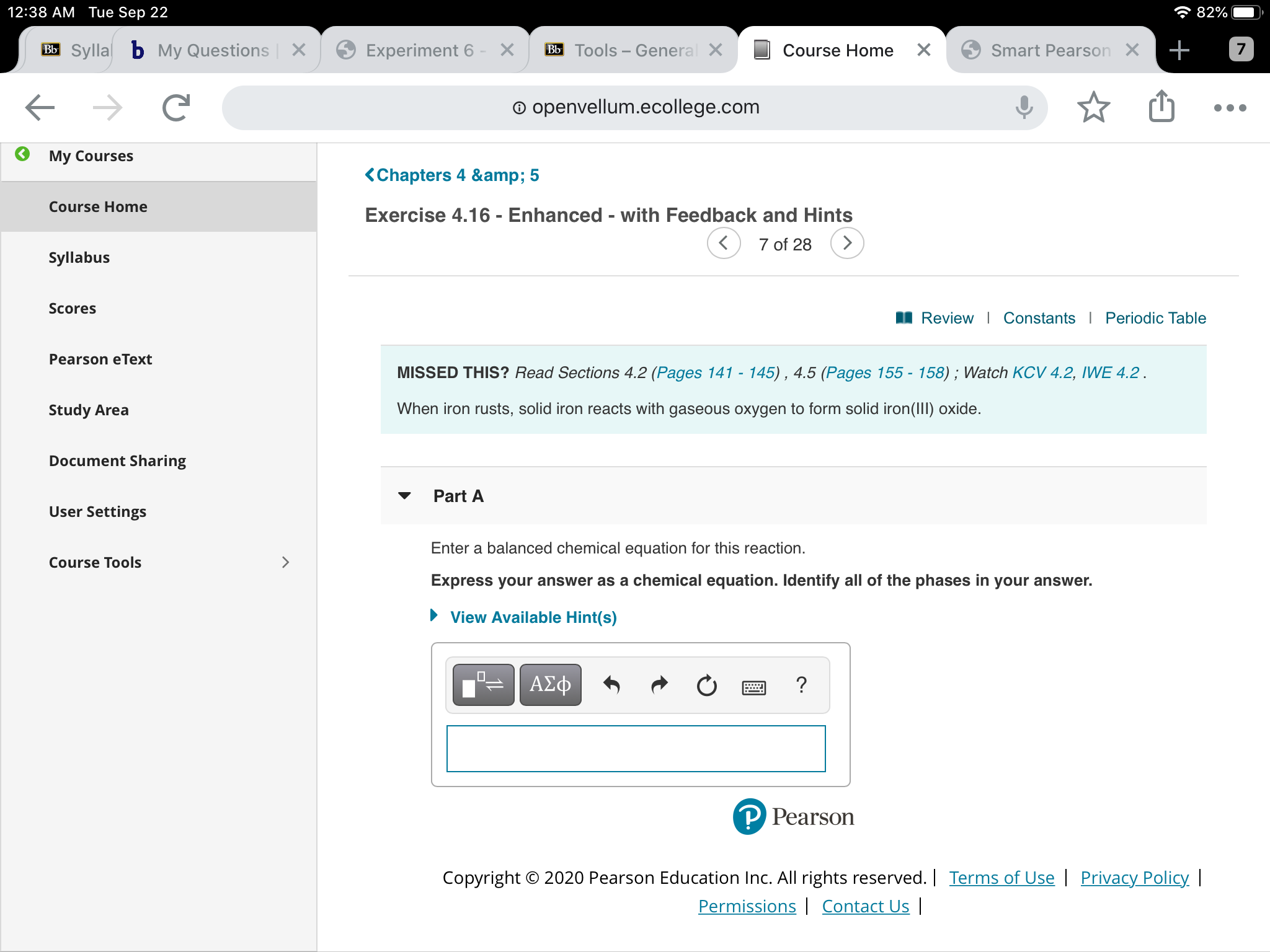
Here is the word equation for the reaction: Enter a balanced chemical equation for this reaction. Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. Afe +302256 b) if 4.0g iron completely reacted, how many. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Web iron reacts with oxygen to form iron (iii) oxide: Iron reacts with oxygen to form iron (iii) oxide. Iron reacts with oxygen at high temperatures to form iron (iii) oxide.
A) write a balanced chemical equation for the reaction. Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described. Enter a balanced chemical equation for this reaction. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Is a specific example of corrosion, which occurs when iron or steel reacts with oxygen and water: Web iron reacts with oxygen to form iron (iii) oxide: Iron + water + oxygen → hydrated iron. Web 3.1 the reaction of iron with oxygen (1 hour) activity: Web iron (iii) oxide is a product of the oxidation of iron.
Solved 3. Iron reacts with oxygen to form iron (III) oxide
Iron reacts with oxygen to form solid iron (iii) oxide. Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. It can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: Iron.
Answered When iron rusts, solid iron reacts with… bartleby
Enter a balanced chemical equation for this reaction. Iron reacts with oxygen to form iron (iii) oxide. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. You know it is iron iii, not iron ii, because oxygen is always. Afe +302256 b) if 4.0g iron completely reacted, how many.
Rusting Equation FOTO IMAGES
It can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: Web iron (iii) oxide is a product of the oxidation of iron. You know it is iron iii, not iron ii, because oxygen is always. A) write a balanced chemical equation for the reaction. Assuming there is an excess.
Spice of Lyfe Chemical Equation For Rusting Of Iron
Web iron reacts with oxygen to form iron (iii) oxide: Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Web ron reacts with oxygen at high temperatures to form iron (iii) oxide. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron.
when iron rusts, solid iron reacts with gaseous oxygen to form solid
Web when two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Is a specific example of corrosion, which occurs when iron or steel reacts with oxygen and water: Web iron (iii) oxide is a product of the oxidation of iron. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2.
Characteristics of iron and its reaction with oxygen MEL Chemistry
Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. Web iron (iii) oxide is a product of the oxidation of iron. Iron + oxygen + water → hydrated iron(iii) oxide Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described. Here is the word equation for the reaction:
PPT The Law of Conservation of Matter PowerPoint Presentation ID
Iron reacts with oxygen at high temperatures to form iron (iii) oxide. Web 3.1 the reaction of iron with oxygen (1 hour) activity: Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. Iron reacts with oxygen to form iron (iii) oxide. Web this reaction can be written in words as iron iii combining with.
[Solved] Solid Iron (III) reacts with oxygen gas to form iron (III
A) write a balanced chemical equation for the reaction. Three different levels of interpretation in science sorting and classifying, interpreting, identifying optional (revision) activity:. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. You know it is iron iii, not iron ii, because oxygen is always. Web ron reacts with oxygen at high temperatures to.
Solved Iron reacts with oxygen to produce iron (III)
Web iron reacts with oxygen to form iron (iii) oxide: Web chemistry chemistry questions and answers write balanced equations for the chemical reactions described. Iron reacts with oxygen to form solid iron (iii) oxide. Upon reacting with oxygen, iron will be oxidized to either the +3. A) write a balanced chemical equation for the reaction.
SOLVEDIron metal reacts with oxygen to give iron…
Afe +302256 b) if 4.0g iron completely reacted, how many. A) write a balanced chemical equation for the reaction. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Assuming there is an excess of iron, how many moles of oxygen. Web when two smaller substances combine to form only.
Iron + Water + Oxygen → Hydrated Iron.
Assuming there is an excess of iron, how many moles of oxygen. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Upon reacting with oxygen, iron will be oxidized to either the +3. Here is the word equation for the reaction:
Three Different Levels Of Interpretation In Science Sorting And Classifying, Interpreting, Identifying Optional (Revision) Activity:.
Enter a balanced chemical equation for this reaction. You know it is iron iii, not iron ii, because oxygen is always. A) write a balanced chemical equation for the reaction. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust.
Web Ron Reacts With Oxygen At High Temperatures To Form Iron (Iii) Oxide.
It can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: 4fe (s)+3o2 (g) 2fe2o3 (s) suppose 13.0 g of iron (fe) is reacted with 19.7 g of oxygen. Web 3.1 the reaction of iron with oxygen (1 hour) activity: Web when two smaller substances combine to form only one product it is usually a synthesis, or combination reaction.
Iron Reacts With Oxygen To Form Iron (Iii) Oxide.
Afe +302256 b) if 4.0g iron completely reacted, how many. 4fe (s)+3o2 (g) 2fe2o3 (s)4fe (s)+3o2 (g) 2fe2o3 (s) suppose 21.2 g21.2 g of. Iron reacts with oxygen to form solid iron (iii) oxide. Web iron reacts with oxygen to form iron (iii) oxide:
:max_bytes(150000):strip_icc()/BalanceEquations1-56a132765f9b58b7d0bcf535.png)