Letairis Enrollment Form
Letairis Enrollment Form - Please complete all felds on this form to prevent any delays in shipment of letairis to. Please complete the following and fax to the number indicated on the form. For women of childbearing potential, (1) a. Web the letairis patient enrollment process. Web required forms for letairis® (ambrisentan): Please complete all felds on this form to prevent any delays in shipment of letairis to. Web to be enrolled into the letairis education and access program, complete and fax the front of this form. Web letairis is a prescription medicine used to treat pulmonary arterial hypertension (pah), which is high blood pressure in the arteries of the lungs. Please complete the following and fax to the number indicated on the form. Web all females must enroll in the letairis rems program to receive letairis.
For women of childbearing potential, (1) a. Web take information on starting a patient on letairis and find rems also leap (the letairis patient support program) enrollments forms. Letairis ® prescription and leap patient support enrollment form. Web step 1 letairis is only available through a certified pharmacy and will be shipped directly to you. Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients who meet the program’s eligibility criteria. (zydus) and cipla usa (cipla) have filed abbreviated new drug applications (andas) to market generic versions of letairis (ambrisentan). Web zydus pharmaceuticals (usa) inc. Letairis can improve your ability. Please complete all felds on this form to prevent any delays in shipment of letairis to. Pregnancy must be excluded prior to the initiation of letairis treatment, monthly.
For women of childbearing potential, (1) a. Letairis ® prescription and leap patient support enrollment form. Web letairis patient enrollment and consent form fax this form and all patient insurance information, including drug benefit cards (front and back), to: Pregnancy must be excluded prior to the initiation of letairis treatment, monthly. Web required forms for ambrisentan: Web required forms for letairis® (ambrisentan): Web the letairis case enrollment process. Female patients of reproductive potential must comply with the pregnancy testing and contraception. Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients who meet the program’s eligibility criteria. Web all female patients must sign an enrollment form.
Letairis (Gilead Sciences, Inc) FDA Package Insert
Web rate the pronunciation difficulty of letairis. Web required forms for letairis® (ambrisentan): Pronunciation of letairis with 2 audio pronunciations. Please complete all felds on this form to prevent any delays in shipment of letairis to. Letairis ® prescription and leap patient support enrollment form.
Patient Enrollment And Consent Form Printable Consent Form
Letairis ® prescription and leap patient support enrollment form. Please complete the following and fax to the number indicated on the form. Web to be enrolled into the letairis education and access program, complete and fax the front of this form. Web letairis patient enrollment and consent form fax this form and all patient insurance information, including drug benefit cards.
Letairis (Gilead Sciences, Inc) FDA Package Insert, Page 8
Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients who meet the program’s eligibility criteria. Web letairis patient enrollment and consent form fax this form and all patient insurance information, including drug benefit cards (front and back), to: Please complete all felds on this form to prevent any delays in shipment of.
Letairis Wins FDA Approval RT
Web letairis patient enrollment and consent form fax this form and all patient insurance information, including drug benefit cards (front and back), to: Please complete the following and fax to the number indicated on the form. Web all females must enroll in the letairis rems program to receive letairis. Pronunciation of letairis with 2 audio pronunciations. Web the letairis patient.
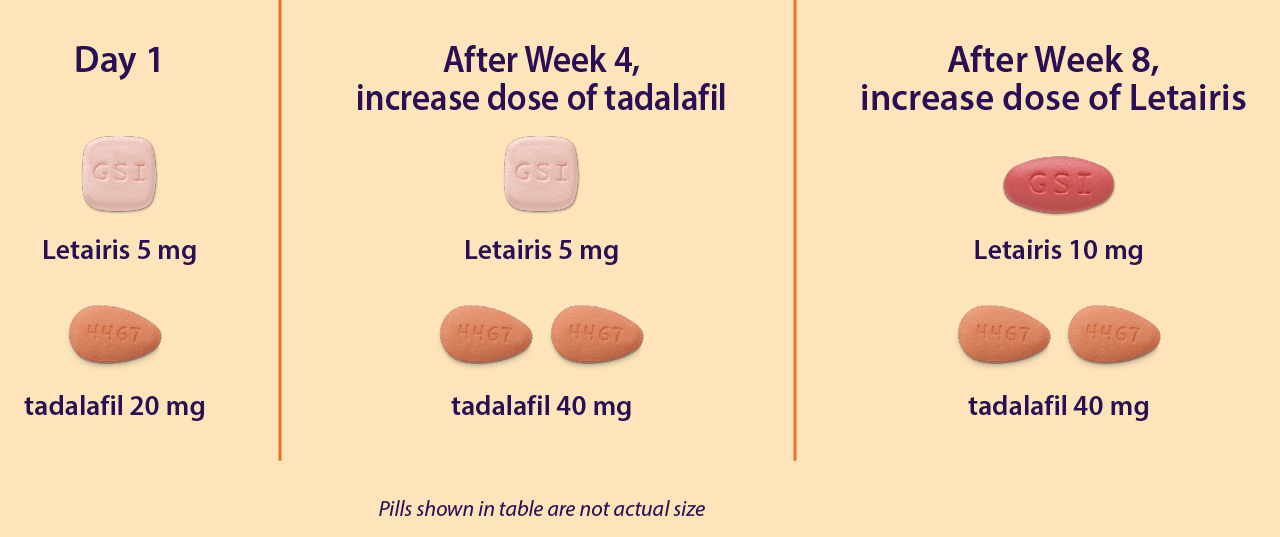
AMBITION Trial Dosing Letairis® (ambrisentan)
For women of childbearing potential, (1) a. Please complete all felds on this form to prevent any delays in shipment of letairis to. Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients who meet the program’s eligibility criteria. Web the letairis case enrollment process. Web rate the pronunciation difficulty of letairis.
Monotherapy Study Letairis® (ambrisentan)
To how a patient on letairis, there be two separate case enrollment forms. Web zydus pharmaceuticals (usa) inc. Pronunciation of letairis with 2 audio pronunciations. Web step 1 letairis is only available through a certified pharmacy and will be shipped directly to you. Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients.
Letairis® (ambrisentan) Official HCP Website
Web zydus pharmaceuticals (usa) inc. To start a patient on letairis, there are two separate patient enrollment mailing. Web required forms for ambrisentan: Please complete the following and fax to the number indicated on the form. Letairis can improve your ability.
medicare macro USA Medicare Plan
Web take information on starting a patient on letairis and find rems also leap (the letairis patient support program) enrollments forms. Please complete the following and fax to the number indicated on the form. Web all female patients must sign an enrollment form. Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients.
Letairis® (ambrisentan) Official Patient Site
Please complete the following and fax to the number indicated on the form. Letairis ® prescription and leap patient support enrollment form. Web required forms for ambrisentan: Web letairis patient enrollment and consent form fax this form and all patient insurance information, including drug benefit cards (front and back), to: To how a patient on letairis, there be two separate.
Rwam Enrollment Form Enrollment Form
To start a patient on letairis, there are two separate patient enrollment mailing. Web rate the pronunciation difficulty of letairis. Web zydus pharmaceuticals (usa) inc. Letairis ® prescription and leap patient support enrollment form. Web the letairis patient enrollment process.
Web The Letairis Patient Enrollment Process.
Web step 1 letairis is only available through a certified pharmacy and will be shipped directly to you. To start a patient on letairis, there are two separate patient enrollment mailing. Web to be enrolled into the letairis education and access program, complete and fax the front of this form. Web zydus pharmaceuticals (usa) inc.
Web Letairis Is A Prescription Medicine Used To Treat Pulmonary Arterial Hypertension (Pah), Which Is High Blood Pressure In The Arteries Of The Lungs.
Pregnancy must be excluded prior to the initiation of letairis treatment, monthly. Web take information on starting a patient on letairis and find rems also leap (the letairis patient support program) enrollments forms. Web all females must enroll in the letairis rems program to receive letairis. Web required forms for letairis® (ambrisentan):
Letairis Can Improve Your Ability.
Work with your pah specialist to enroll in the leap patient support program. Web the letairis case enrollment process. Web letairis patient enrollment and consent form fax this form and all patient insurance information, including drug benefit cards (front and back), to: Please complete all felds on this form to prevent any delays in shipment of letairis to.
Pronunciation Of Letairis With 2 Audio Pronunciations.
Please complete the following and fax to the number indicated on the form. Please complete all felds on this form to prevent any delays in shipment of letairis to. Web referral to the patient assistance solutions™ program, which provides letairis at no cost for qualified patients who meet the program’s eligibility criteria. (zydus) and cipla usa (cipla) have filed abbreviated new drug applications (andas) to market generic versions of letairis (ambrisentan).