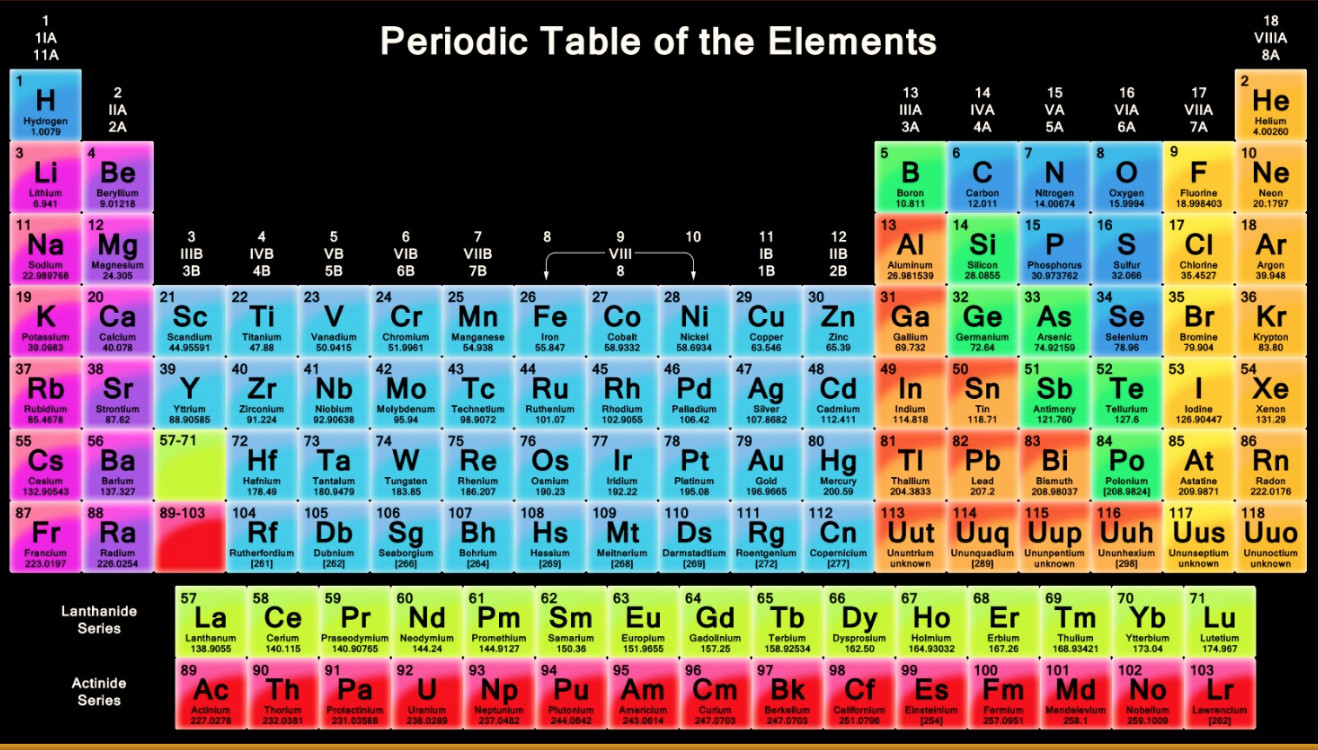
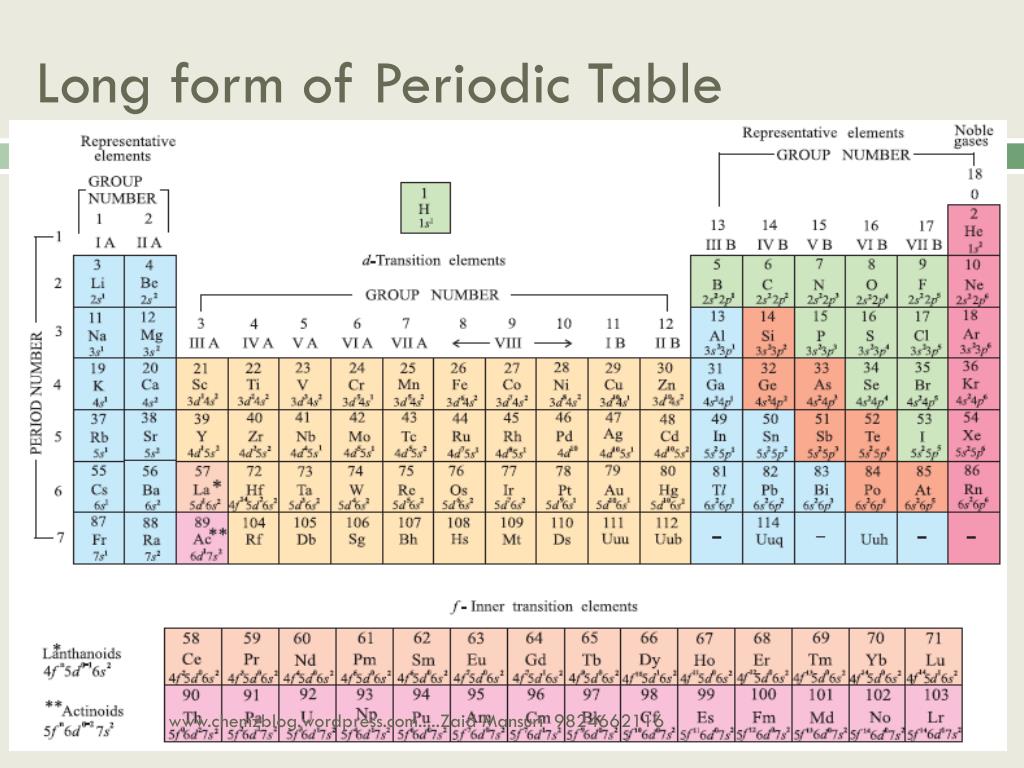
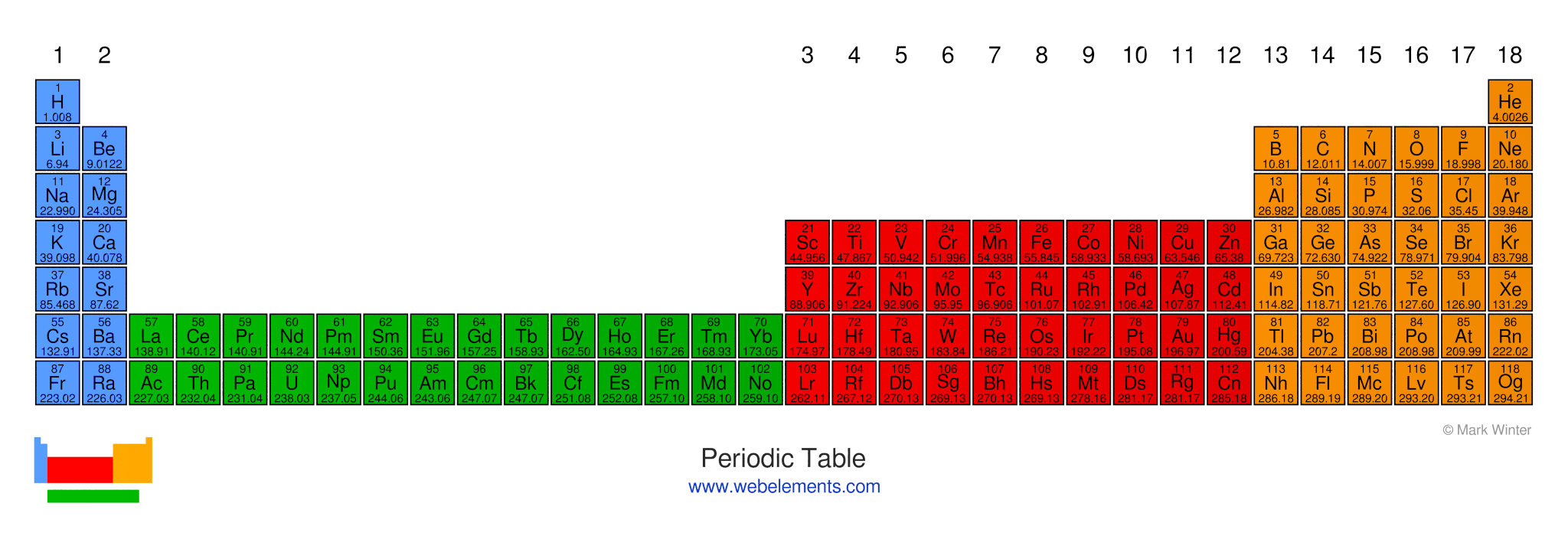
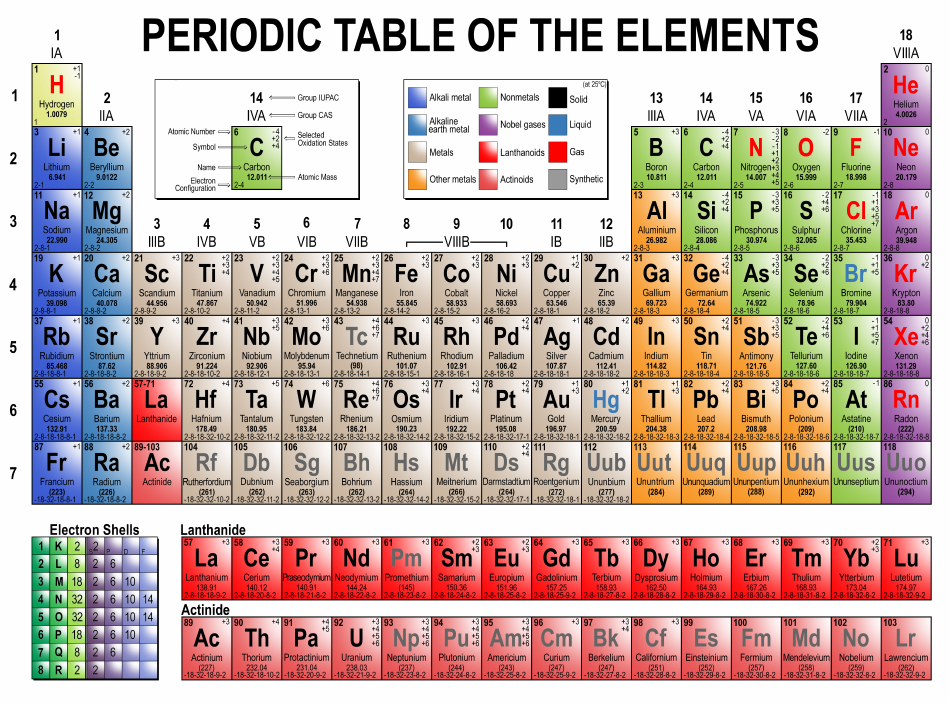
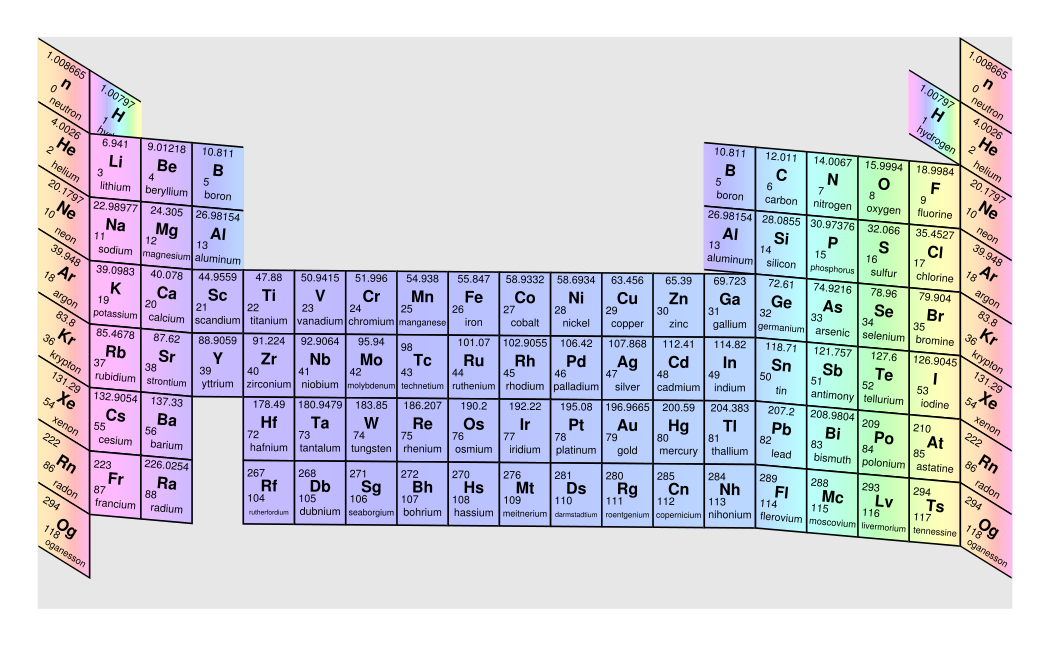
Long Form Of The Periodic Table
Long Form Of The Periodic Table - Periodic_table_long_form.pdf — pdf document, 625 kb (640888 bytes) All the inner shells are filled, only the outermost shell is incomplete. As of now, 118 elements are present in periodic table. In this table, the elements are arranged in increasing order according to their atomic numbers. (ii) it relates the position of an element in the periodic table with its electronic configuration. As of july 2023, the element with the highest atomic number known is oganesson ( z = 118), which completes the seventh period (row) in the periodic table. Elements of each group have the same number of valence electrons, so they have similar properties. In the long form of periodic table the total number of groups are. Web this is a list of the 118 chemical elements that have been identified as of 2023. It includes group 1, 2 and group 13 to 17.
Web both forms represent the same periodic table. It is made up of 18 vertical columns and 7 horizontal rows. Web solution verified by toppr correct option is a) the long form of periodic table is based on atomic number. Then in 1869, 69 elements were found that time. (ii) it relates the position of an element in the periodic table with its electronic configuration. (2) there are 18 vertical columns known as groups. He developed table contain only 28 elements, which are classified on the basis of valence, mendeleev: The table is an arrangement of elements in increasing atomic number order. Mention the number of elements present in each of the periods in the long form periodic table. Atomic number is equal to number of electrons.
Mention the number of elements present in each of the periods in the long form periodic table. For mg, the maximum principal quantum number is 3, thus it belongs to period 3 of the periodic table. All elements in the eighth period and beyond thus remain purely. The table is the arrangement of elements in increasing order of their atomic numbers. Web solution verified by toppr correct option is d) lotharmeyer: Web the long form periodic table has been the following merits: (c) maximum principal quantum number of any element of the period since each period starts with the filling of electrons in a new principal quantum number, therefore, the period number in the long form of the periodic table refers to the maximum principal quantum number of any element in the period. All the inner shells are filled, only the outermost shell is incomplete. He developed the periodic table which we used today, on the basis of atomic number. (ii) it relates the position of an element in the periodic table with its electronic configuration.
Modern Periodic Table Periodic Classification of Elements
Periodic_table_long_form.pdf — pdf document, 625 kb (640888 bytes) It includes group 1, 2 and group 13 to 17. He developed table contain only 28 elements, which are classified on the basis of valence, mendeleev: The table is the arrangement of elements in increasing order of their atomic numbers. All elements in the eighth period and beyond thus remain purely.
PPT CHAPTER 3 PERIODIC CLASSIFICATION PowerPoint Presentation, free
(2) there are 18 vertical columns known as groups. (ii) it relates the position of an element in the periodic table with its electronic configuration. He developed table contain only 28 elements, which are classified on the basis of valence, mendeleev: Web solution verified by toppr correct option is d) lotharmeyer: It includes group 1, 2 and group 13 to.
Long form of periodic table Download Scientific Diagram
(i) long form periodic table is based upon the atomic numbers which is more fundamental properties of the atom. For mg, the maximum principal quantum number is 3, thus it belongs to period 3 of the periodic table. The table is an arrangement of elements in increasing atomic number order. Hence correct answer is option a. Arranged elements by atomic.
The periodic table of the elements by WebElements
Arranged elements by atomic mass in his periodic table. Web periodic table of elements. (2) there are 18 vertical columns known as groups. Elements of each group have the same number of valence electrons, so they have similar properties. Web main features of the long form of the periodic table :
Long Form Periodic Table of Elements Download Printable PDF
Web solution verified by toppr correct option is b) correct option: Web main features of the long form of the periodic table : Hence correct answer is option a. He developed the periodic table which we used today, on the basis of atomic number. It consists of 18 vertical columns and 7 horizontal rows.
😀 Long form of periodic table. Useful Notes on the Merits and Defects
(3) there are 7 horizontal rows known as periods. In the long form periodic table all elements have been arranged in the increasing order of atomic numbers whereas table have been arranged in the increasing order of atomic masses. It determines the period number of the element. Periodic_table_long_form.pdf — pdf document, 625 kb (640888 bytes) He developed table contain only.
Pin on Glaze it up
(c) maximum principal quantum number of any element of the period since each period starts with the filling of electrons in a new principal quantum number, therefore, the period number in the long form of the periodic table refers to the maximum principal quantum number of any element in the period. Web the long form periodic table has been the.
Free online, printable periodic tables. Tons of them! Great resource
And in 2019, 118 elements were found. Long form periodic table is regarded as letter than the mendellev's table due to the following reasons: Elements of each group have the same number of valence electrons, so they have similar properties. He developed the periodic table which we used today, on the basis of atomic number. Web solution verified by toppr.
Periodic Table, LongForm, 50" x 38" UniScience Laboratories
Web if the long form of periodic table the total number of periods is. (ii) it relates the position of an element in the periodic table with its electronic configuration. Web both forms represent the same periodic table. Web periodic table of elements. It consists of 18 vertical columns and 7 horizontal rows.
Which block of the periodic table is the largest? Socratic
Web long form of periodic table history of periodic table in 1800, only 30 elements were discovered that time. He developed table contain only 28 elements, which are classified on the basis of valence, mendeleev: Hence correct answer is option a. Web the long form of the periodic table. And in 2019, 118 elements were found.
Web Both Forms Represent The Same Periodic Table.
Web main features of the long form of the periodic table : Web long form of periodic table history of periodic table in 1800, only 30 elements were discovered that time. The table is the arrangement of elements in increasing order of their atomic numbers. It includes group 1, 2 and group 13 to 17.
The 18 Vertical Columns Are Named For Numbers As 1, 2, 3.
According to time, this invention was increased and 56 elements were found in 1865. Web solution verified by toppr correct option is d) lotharmeyer: Web an extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven. (i) long form periodic table is based upon the atomic numbers which is more fundamental properties of the atom.
Elements Of Each Group Have The Same Number Of Valence Electrons, So They Have Similar Properties.
Web if the long form of periodic table the total number of periods is. Mention the number of elements present in each of the periods in the long form periodic table. In the long form periodic table all elements have been arranged in the increasing order of atomic numbers whereas table have been arranged in the increasing order of atomic masses. It determines the period number of the element.
All Elements In The Eighth Period And Beyond Thus Remain Purely.
(iii) it removes all the anomalies and drawbacks of the. (c) maximum principal quantum number of any element of the period since each period starts with the filling of electrons in a new principal quantum number, therefore, the period number in the long form of the periodic table refers to the maximum principal quantum number of any element in the period. Then in 1869, 69 elements were found that time. Arranged elements by atomic mass in his periodic table.