Manganese Electron Configuration Long Form
Manganese Electron Configuration Long Form - Which is abundant in nature, has long been used as a pigment. Thus answer is option c. It was recognized as an element in 1774 by the swedish chemist carl wilhelm scheele. For determining its electron configuration, we first look at its atomic number to find out the. Compared to 1953, levels of. Web what is the electron configuration for manganese? Web the electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. Web the complete electron configuration of manganese is 1s2 2s 2 2p6 3s2 3p6 4s23d5.manganese has 7 valence electrons around the nucleus and its atomic number is 25. It is found in nature as a free element, often combined with iron and other minerals. Therefore, the valence electrons of manganese are seven.
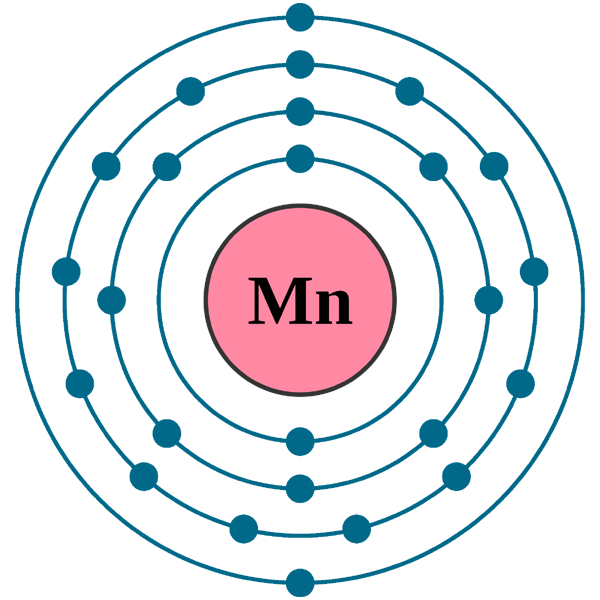
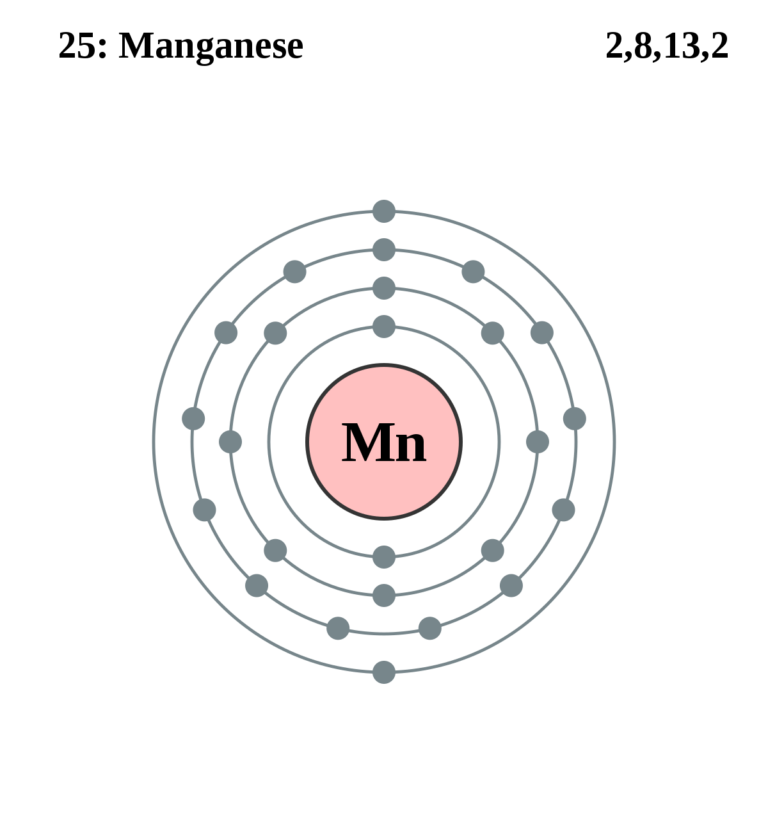
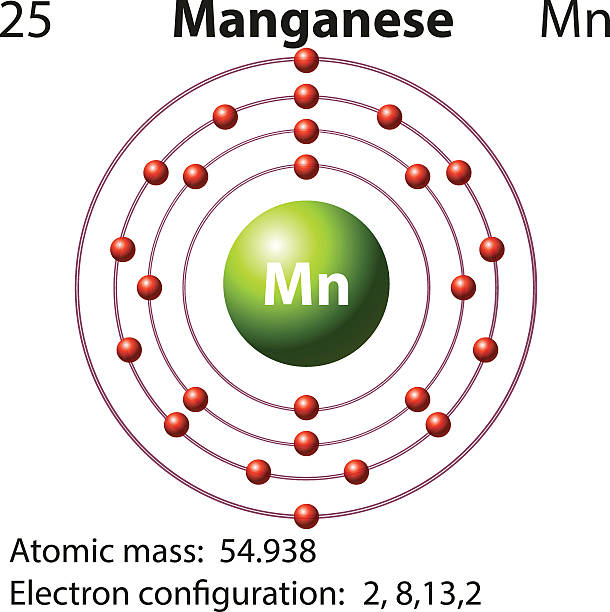
Electron configuration of oxygen (o. The atomic number of each element increases by one, reading from left to right. Fuels containing manganese tend to form manganese carbides, which damage exhaust valves. Web today in this video, we will help you determine the electron configuration for the manganese element. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Possible oxidation states are +2,3,4,7. The chemical symbol for manganese is mn. Members of a group typically have similar properties and electron configurations in their outer shell. Web in the periodic table, the elements are listed in order of increasing atomic number z. Web the electron configuration of manganese is 1s2 2s2 2p6 3s2 3p6 3d5 4s2.
Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. The most common oxidation states of manganese are +2, +3, +4, +6, and +7, though all oxidation states from −3 to +7 have been observed. Web jan 15, 2016 [ar]4s23d5 explanation: The kossel shell structure of manganese. Although rarely used in pure form, manganese is. It was recognized as an element in 1774 by the swedish chemist carl wilhelm scheele. Mn has an atomic number of 25. Web the complete electron configuration of manganese is 1s2 2s 2 2p6 3s2 3p6 4s23d5.manganese has 7 valence electrons around the nucleus and its atomic number is 25. Web manganese ( i) has the same electron configuration as that of iron ( ii ), but until now has typically been overlooked in the search for cheap mlct luminophores. Electron configuration of oxygen (o.
Manganese Element Electron Configuration imgsnicker
Electron configuration of boron (b) [he] 2s 2 2p 1: Electron configuration and oxidation states of manganese. Members of a group typically have similar properties and electron configurations in their outer shell. Thus answer is option c. Web ← electronic configurations of elements mn (manganese) is an element with position number 25 in the periodic table.
Dictionary by WeiJin Tang (湯偉晉 編寫的字典) 十二月 2010
Simply use this information to obtain its electronic configuration. For determining its electron configuration, we first look at its atomic number to find out the. Web ← electronic configurations of elements mn (manganese) is an element with position number 25 in the periodic table. Mn has an atomic number of 25. Web electron configuration 3d 5 4s 2:
Manganese electron configuration Newton Desk
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Period a horizontal row in the periodic table. Thus answer is option c. Electron configuration of oxygen (o.
Manganese Electron Configuration Illustrations, RoyaltyFree Vector
Electron configuration of oxygen (o. Web electron configuration 3d 5 4s 2: Mn has an atomic number of 25. Web the electron configuration of manganese is 1s2 2s2 2p6 3s2 3p6 3d5 4s2. Web what is the electron configuration for manganese?
Manganese Iv Ion Electron Configuration The ground state electron
The most common oxidation states of manganese are +2, +3, +4, +6, and +7, though all oxidation states from −3 to +7 have been observed. Web the atomic number of manganese (mn) is 25. Electron configuration of oxygen (o. 1s 2 2s 2 2p 3: Possible oxidation states are +2,3,4,7.
Electron Configuration For Manganese Atomic Number 25 How Do You Draw
Therefore, the valence electrons of manganese are seven. For determining its electron configuration, we first look at its atomic number to find out the. These materials are called ferromanganese. The ground state electron configuration of ground state gaseous neutral manganese is [ ar ]. Web in the periodic table, the elements are listed in order of increasing atomic number z.
Manganese Electron Configuration Manganese Orbital Diagram Insight
Therefore, the valence electrons of manganese are seven. For determining its electron configuration, we first look at its atomic number to find out the. The principal ore of manganese is pyrolusite, mno 2, which occurs in black, massive forms. Electron configuration and oxidation states of manganese. Web the electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2.
Symbol of Manganese Archives Dynamic Periodic Table of Elements and
Web manganese, chemical element that is a silvery white, hard, brittle metal of group 7 in the periodic table. Web manganese is also known to to lie on the ocean floor in the form of nodules, which are large lumps of metallic ores. It is found in nature as a free element, often combined with iron and other minerals. Web.
Manganese Ion Electron Configuration Solved Manganese Is Found As
Web the complete electron configuration of manganese is 1s2 2s 2 2p6 3s2 3p6 4s23d5.manganese has 7 valence electrons around the nucleus and its atomic number is 25. Physical properties the atomic structure of manganese includes four electron subshells. Thus its electronic configuration is [ar]4s 23d 5 or 1s 22s 22p 63s 23p 64s 24d 5. Web this page shows.
Manganese Electron Configuration Illustrations, RoyaltyFree Vector
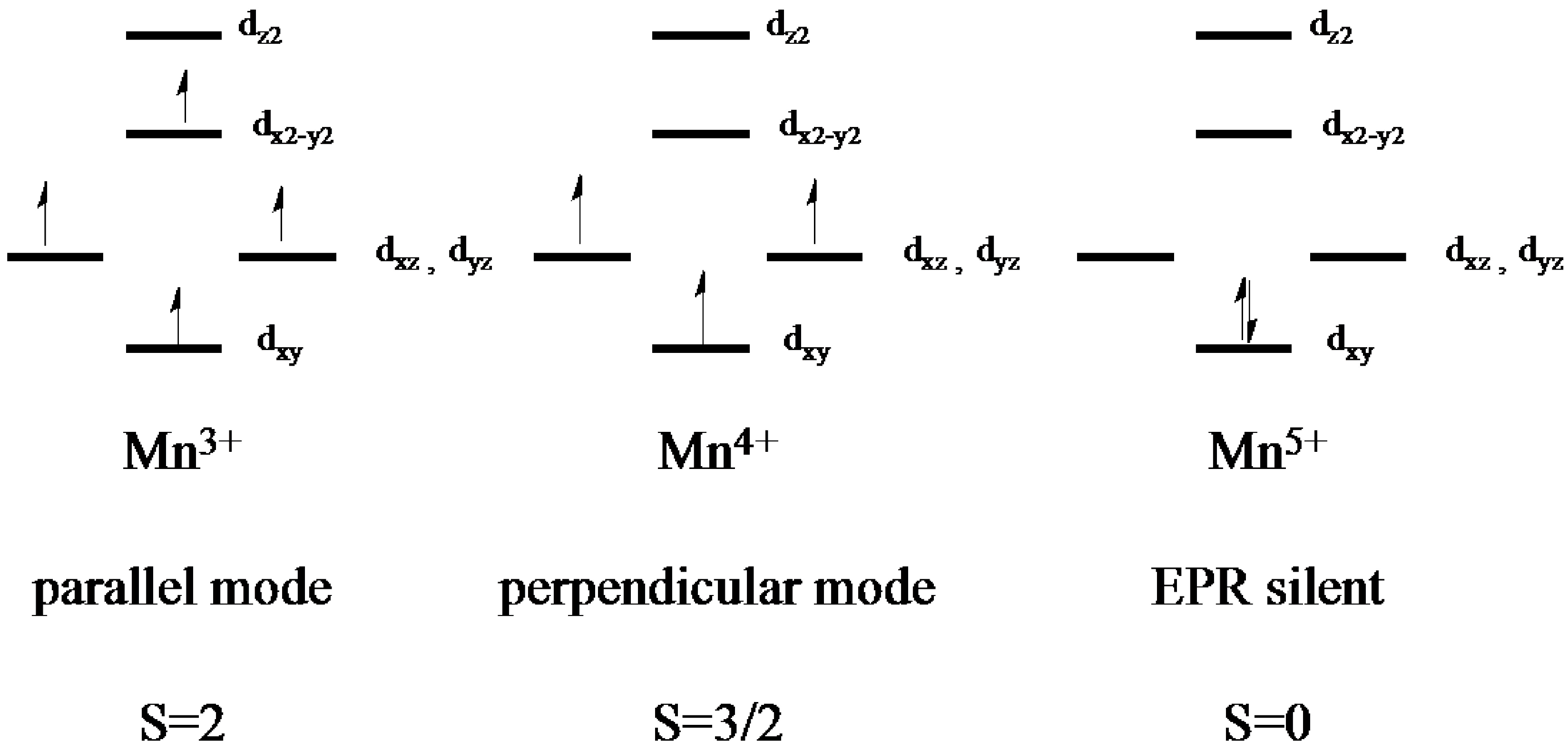
Web manganese ion (mn 2+, mn 3+, mn 4+) electron configuration. Electronic configuration of the manganese atom in ascending order of orbital energies: The kossel shell structure of manganese. It is found in nature as a free element, often combined with iron and other minerals. Web the electron configuration of manganese is 1s2 2s2 2p6 3s2 3p6 3d5 4s2.
Web The Electron Configuration Of Manganese Is 1S2 2S2 2P6 3S2 3P6 3D5 4S2.
1s 22s 22p 63s 23p 64s 24d 5. Schematic electronic configuration of manganese. The ground state electron configuration of ground state gaseous neutral manganese is [ ar ]. The principal ore of manganese is pyrolusite, mno 2, which occurs in black, massive forms.
Web Either Way, The Manganese Electron Configuration Will Be 1S2 2S2 2P6 3S2 3P6 3D5 4S2 Note That When Writing The Electron Configuration For An Atom Like Mn, The 3D Is Usually Written Before The 4S.
For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Therefore, the valence electrons of manganese are seven. 1s 2 2s 2 2p 2: Web electron configuration 3d 5 4s 2:
Simply Use This Information To Obtain Its Electronic Configuration.
Thus its electronic configuration is [ar]4s 23d 5 or. Manganese is the chemical element of the periodic table of elements located in group 7, its symbol is mn and its atomic number is 25. Which is abundant in nature, has long been used as a pigment. Although rarely used in pure form, manganese is.
The Most Common Oxidation States Of Manganese Are +2, +3, +4, +6, And +7, Though All Oxidation States From −3 To +7 Have Been Observed.
Its principal use is in the manufacture of alloy steel. The distribution of electrons is as 2 electrons in 1s subshell, 2 electrons in 2s subshell, 6 electrons in 2p subshell, 2 electrons in 3s, 6 electrons in 3p, 2 electrons in 4s and 5. Members of a group typically have similar properties and electron configurations in their outer shell. 4s2 and the term symbol is 6s5/2.