Ponvory Start Form
Ponvory Start Form - Dosage form and strengths for ponvory. Ad learn what to discuss with your healthcare provider before starting vumerity®. Selective immunosuppressants medically reviewed by drugs.com. Web start ponvory in patients with active infection. 5.0 mm 2 on one side and an arch. Web overdose ask your doctor what is ponvory? Obtain a complete blood count (cbc) before initiating treatment. Explore more information on the patient site. Your starter pack is clearly labeled with the day you will take. Tablet, film coated drug class:
Web cigna covers ponesimod (ponvory™) as medically necessary when the following criteria are met for fda indications or other uses with supportive evidence: Ponvory may increase the risk of infections. Dosage form and strengths for ponvory. 5.0 mm 2 on one side and an arch. Web the ponvory® starter pack slowly increases your dose of ponvory® so your body can adjust to the new medicine. If you have certain forms of multiple sclerosis (ms), you may be interested in learning more about ponvory. Based on analysis by drugpatentwatch, the earliest date for a generic. See what vumerity® could do for you. Explore more information on the patient site. Only start your treatment with ponvory using the starter pack.
Dosage form and strengths for ponvory. Web ponvory is protected by five us patents and one fda regulatory exclusivity. Explore more information on the patient site. Your starter pack is clearly labeled with the day you will take. Based on analysis by drugpatentwatch, the earliest date for a generic. Ad learn what to discuss with your healthcare provider before starting vumerity®. Web watch newsmax live for the latest news and analysis on today's top stories, right here on facebook. Web start ponvory in patients with active infection. (5.1) • bradyarrhythmia and atrioventricular conduction delays: Web overdose ask your doctor what is ponvory?
US FDA approves Janssen’s PONVORY for relapsing multiple sclerosis
Web ponvory, which comes in tablet form, is taken orally, once daily. Web ponvory is a prescription medicine that is used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing remitting disease,. Web your heart called an electrocardiogram (ecg) before you take your first dose of ponvory. Only start your treatment with ponvory using the starter.
FDA Approves Ponvory Anton RX Report Anton Health
See what vumerity® could do for you. See what vumerity® could do for you. Web start ponvory in patients with active infection. Web the ponvory® starter pack slowly increases your dose of ponvory® so your body can adjust to the new medicine. Selective immunosuppressants medically reviewed by drugs.com.
Ponvory FDA prescribing information, side effects and uses
Only start your treatment with ponvory using the starter pack. Explore more information on the patient site. Dosage form and strengths for ponvory. It is thought to act by promoting the retention of certain white blood cells in the body’s lymph. See what vumerity® could do for you.
PONVORY Dosage & Rx Info Uses, Side Effects
Explore more information on the patient site. Web your heart called an electrocardiogram (ecg) before you take your first dose of ponvory. Based on analysis by drugpatentwatch, the earliest date for a generic. Web watch newsmax live for the latest news and analysis on today's top stories, right here on facebook. Ponvory may increase the risk of infections.
Your starter pack is clearly labeled with the day you will take. Web ponvory is protected by five us patents and one fda regulatory exclusivity. Dosage form and strengths for ponvory. Ad learn what to discuss with your healthcare provider before starting vumerity®. Ad learn what to discuss with your healthcare provider before starting vumerity®.
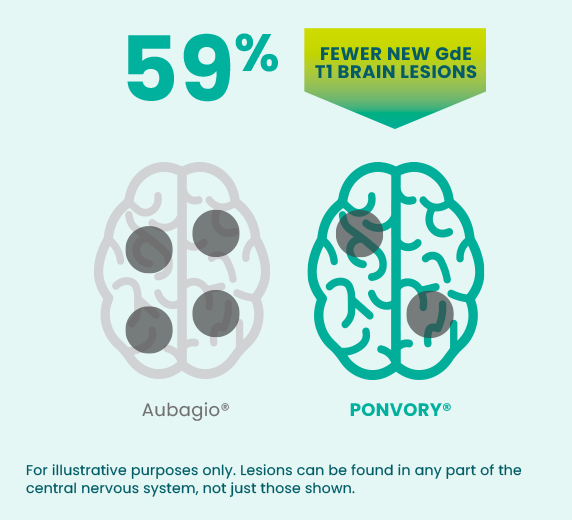
59 fewer new GdE T1 brain lesions
It is thought to act by promoting the retention of certain white blood cells in the body’s lymph. Explore more information on the patient site. Ad learn what to discuss with your healthcare provider before starting vumerity®. See what vumerity® could do for you. Ponvory may result in a transient decrease in heart rate;
Ponvory FDA prescribing information, side effects and uses
Ponvory may result in a transient decrease in heart rate; 5.0 mm 2 on one side and an arch. Explore more information on the patient site. Web start ponvory in patients with active infection. Ad learn what to discuss with your healthcare provider before starting vumerity®.
Start Form Pdf Fill Out and Sign Printable PDF Template signNow
(5.1) • bradyarrhythmia and atrioventricular conduction delays: If you have certain forms of multiple sclerosis (ms), you may be interested in learning more about ponvory. Only start your treatment with ponvory using the starter pack. Web ponvory, which comes in tablet form, is taken orally, once daily. 5.0 mm 2 on one side and an arch.
Newly Approved MS Drug Ponvory Reduces Fatigue, Study Shows Everyday
Web overdose ask your doctor what is ponvory? Explore more information on the patient site. Web ponvory is a prescription medicine that is used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing remitting disease,. Web your heart called an electrocardiogram (ecg) before you take your first dose of ponvory. Web ponvory is protected by five.
Ponvory Another Drug for Multiple Sclerosis BioPharma Media
It is thought to act by promoting the retention of certain white blood cells in the body’s lymph. (5.1) • bradyarrhythmia and atrioventricular conduction delays: Ponvory may increase the risk of infections. See what vumerity® could do for you. Explore more information on the patient site.
Web The Ponvory® Starter Pack Slowly Increases Your Dose Of Ponvory® So Your Body Can Adjust To The New Medicine.
Ad learn what to discuss with your healthcare provider before starting vumerity®. Web overdose ask your doctor what is ponvory? A phase 3 clinical trial involving more than 1,100 people with relapsing ms compared daily oral ponvory to oral aubagio. Ad learn what to discuss with your healthcare provider before starting vumerity®.
Web Janssen Link For Ponvory™ Enables Eligible Patients To Receive Ponvory™ (Ponesimod) At No Cost Until They Receive Coverage Or For Up To 24 Months From Program.
Ponvory may increase the risk of infections. Based on analysis by drugpatentwatch, the earliest date for a generic. It is thought to act by promoting the retention of certain white blood cells in the body’s lymph. Explore more information on the patient site.
Selective Immunosuppressants Medically Reviewed By Drugs.com.
Dosage form and strengths for ponvory. Web ponvory is a prescription medicine that is used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing remitting disease,. Web your heart called an electrocardiogram (ecg) before you take your first dose of ponvory. Tablet, film coated drug class:
Obtain A Complete Blood Count (Cbc) Before Initiating Treatment.
5.0 mm 2 on one side and an arch. See what vumerity® could do for you. Web cigna covers ponesimod (ponvory™) as medically necessary when the following criteria are met for fda indications or other uses with supportive evidence: Your starter pack is clearly labeled with the day you will take.