States Of Matter Chapter 10 Review
States Of Matter Chapter 10 Review - Web hsms2k review on states of matter vocabulary terms in this set (43) kinetic molecular theory based on the idea that particles of matter are always in motion ideal gas a hypothetical gas that perfectly fits. Click the card to flip 👆. 99 , and 1 person voted. Click the card to flip 👆. Assume that the best path in this case is the path. 3.97 avg rating — 28,463 ratings. Web learn about the definition of the kinetic theory of matter, phase changes, and the four states of matter. Find other quizzes for chemistry and more on quizizz for free! Click the card to flip 👆. Which one of these could be described as having high density and a definite volume?
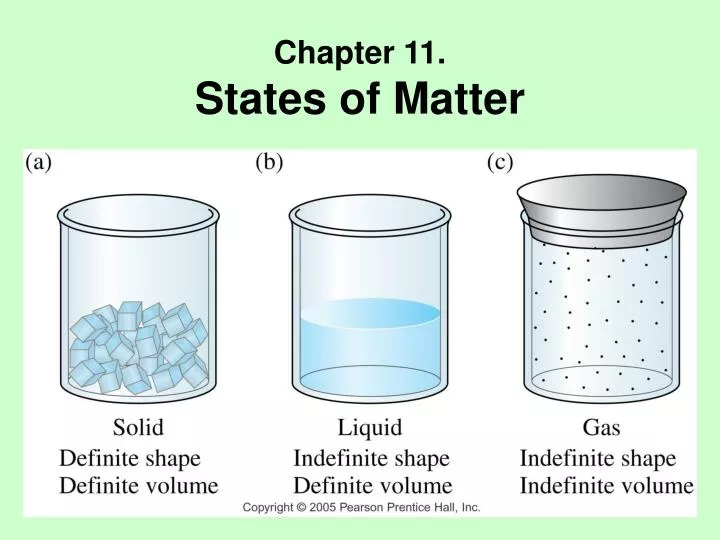
Chapter 10 states of matter test answers. Based on idea that particles of matter are always in motion. Web holt mcdougal modern chemistry 1 states of matter chapter 10 review states of matter section 2 short answer answer the following questions in the space provided. Solid liquid gas definite volume definite volume indefinite volume definite shape indefinite shape indefinite shape strong intermolecular (attractive) forces moderately strong. Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the crystal covalent network crystal typically has the lowest melting point of. Define solid, liquid, and gas. Click let’s review to review. Click the card to flip 👆. How does the motion of particles change as a substance. Click the card to flip 👆.
Web what state of matter is segment 5? (d) the ability to change to a gas. Web chapter 10 review states of matter (section 2) 5.0 (4 reviews) liquids possess all the following properties except: _____ liquids possess all the following. Click the card to flip 👆. Click the card to flip 👆. How does the motion of particles change as a substance. States of matter quiz for 9th grade students. Based on idea that particles of matter are always in motion. Define solid, liquid, and gas.
Unit 2 Review States of Matter Crossword WordMint
Physical property of matter that indicates weather a sample of matter is a solid, liquid, gas, or plasma. Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the crystal covalent network crystal typically has the lowest melting point of. Chapter 10 states of matter.
PPT Chapter 10 States of Matter PowerPoint Presentation, free
How does the motion of particles change as a substance. Web holt mcdougal modern chemistry 1 states of matter chapter 10 review states of matter section 2 short answer answer the following questions in the space provided. Click let’s review to review. Chem chapter 10 review draft. (b) the ability to diffuse.
Wild Adventures October 2013
Chapter 10 states of matter test answers. Web comparing the states of matter; Matter with a definite shape and volume. Chapter 10 states of matter test answers. Assume that the best path in this case is the path.
PPT Chapter 11. States of Matter PowerPoint Presentation, free
Web chapter 10 review states of matter (section 2) 5.0 (4 reviews) liquids possess all the following properties except: Matter with a definite shape and volume. (d) the ability to change to a gas. Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the.
PPT Chapter 10 States of Matter PowerPoint Presentation, free
Web holt mcdougal modern chemistry 1 states of matter chapter 10 review states of matter section 2 short answer answer the following questions in the space provided. Chem chapter 10 review draft. How does the motion of particles change as a substance. Chapter 10 states of matter test answers. Click let’s review to review.
States of Matter
Chapter 10 states of matter test answers. (b) the ability to diffuse. Assume that a, b, c, d, and e are autonomous systems (ass). The heart of the matter. Web comparing the states of matter;
Chapter 10 states of matter
Chem chapter 10 review draft. (d) the ability to change to a gas. Chapter 10 states of matter test answers. States of matter quiz for 9th grade students. Compare and contrast the proximities and mobilities of solid, liquid, and gaseous particles.
Chapter 3 States of Matter
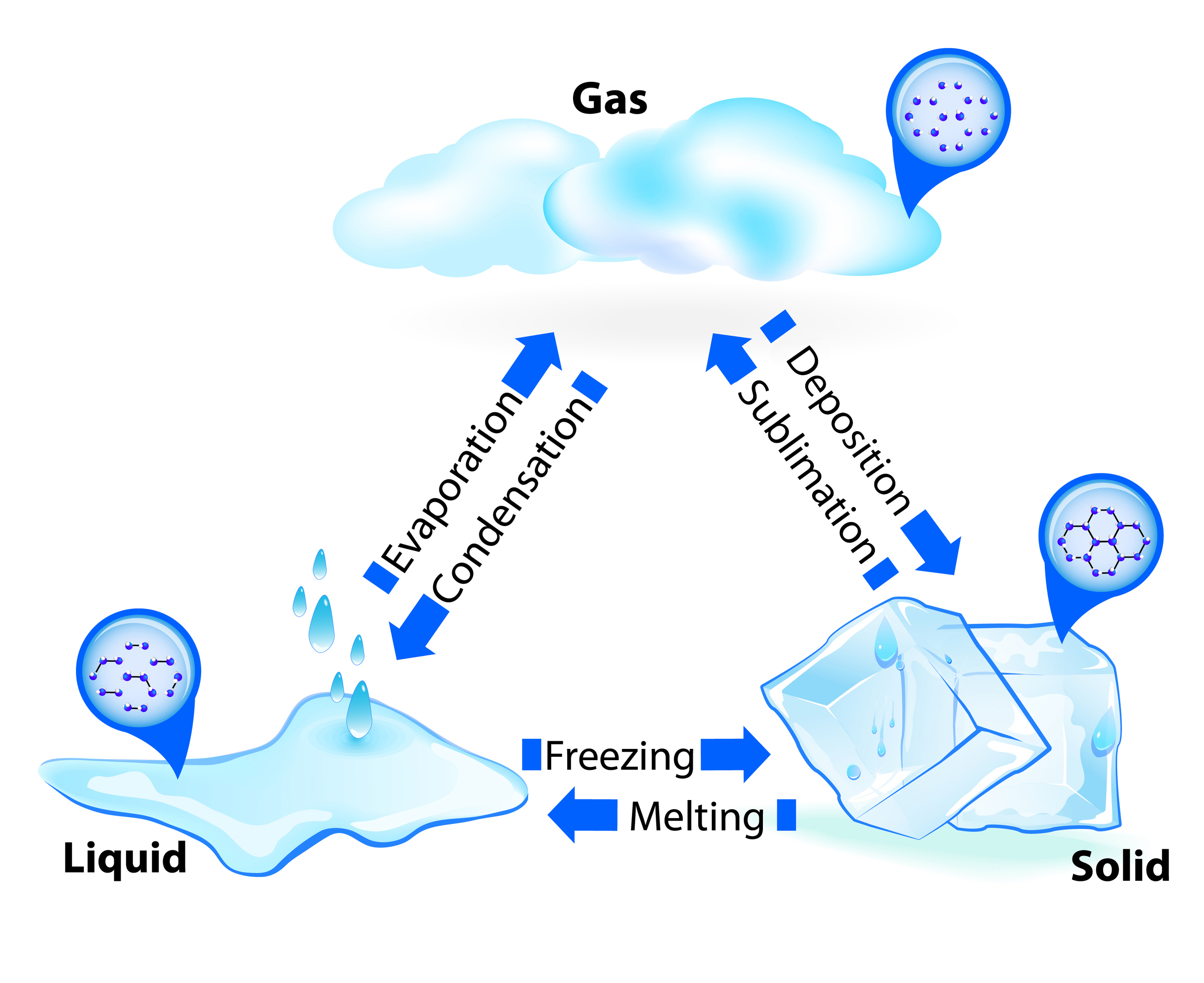
Web section review worksheets answer key chapter 10 review worksheet answer key states of matter pp phase changes pp Chapter 10 states of matter test answers. Assume that the best path in this case is the path. Web what state of matter is segment 5? How does the motion of particles change as a substance.
States of Matter
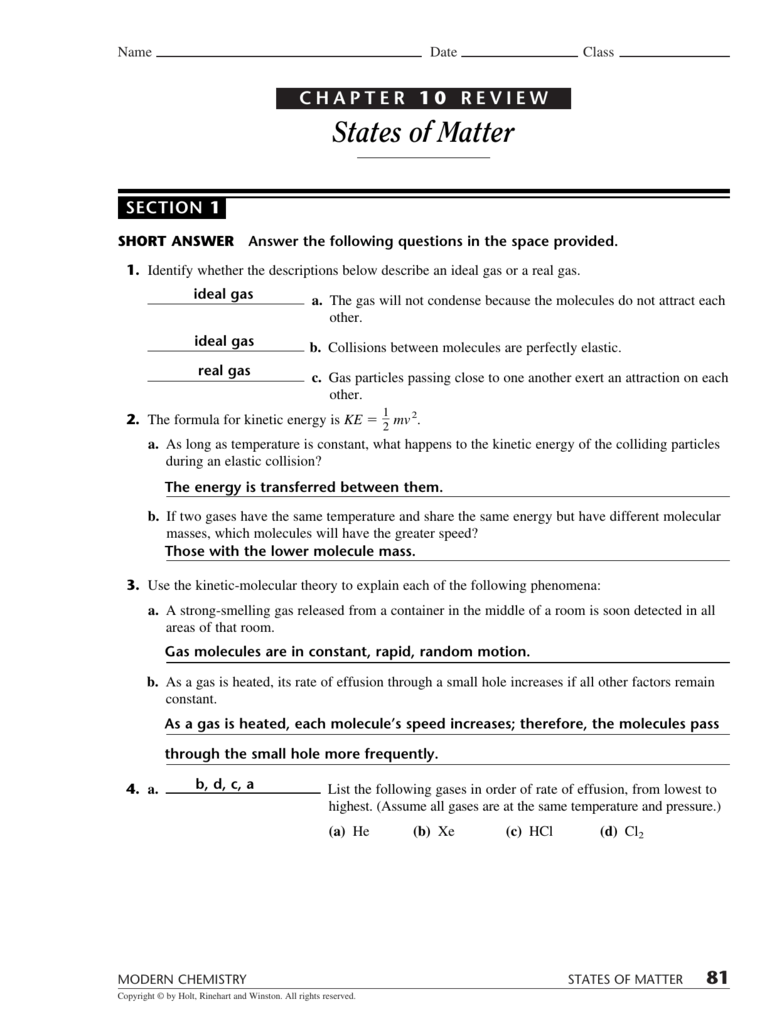
Find the path vector for each as using the algorithm in table 20.3. Click the card to flip 👆. Web chapter 10 review states of matter (section 1) 4.3 (9 reviews) the gas will not condense because the molecules do not attract each other. 99 , and 1 person voted. Physical property of matter that indicates weather a sample of.
Find The Path Vector For Each As Using The Algorithm In Table 20.3.
Chapter 10 states of matter test answers. (b) the ability to diffuse. 99 , and 1 person voted. Web comparing the states of matter;
Web Chapter 10 Review States Of Matter (Section 2) 5.0 (4 Reviews) Liquids Possess All The Following Properties Except:
Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the crystal covalent network crystal typically has the lowest melting point of. Compare and contrast the proximities and mobilities of solid, liquid, and gaseous particles. _____ liquids possess all the following. (d) the ability to change to a gas.
Chem Chapter 10 Review Draft.
Download chapter 10 states of matter test answers: Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. Which one of these could be described as having high density and a definite volume? Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter.
How Does The Motion Of Particles Change As A Substance.
Click the card to flip 👆. Web section review worksheets answer key chapter 10 review worksheet answer key states of matter pp phase changes pp Solid liquid gas definite volume definite volume indefinite volume definite shape indefinite shape indefinite shape strong intermolecular (attractive) forces moderately strong. Ideal gas molecules do not repel or.