When Energy Is Converted From One Form To Another
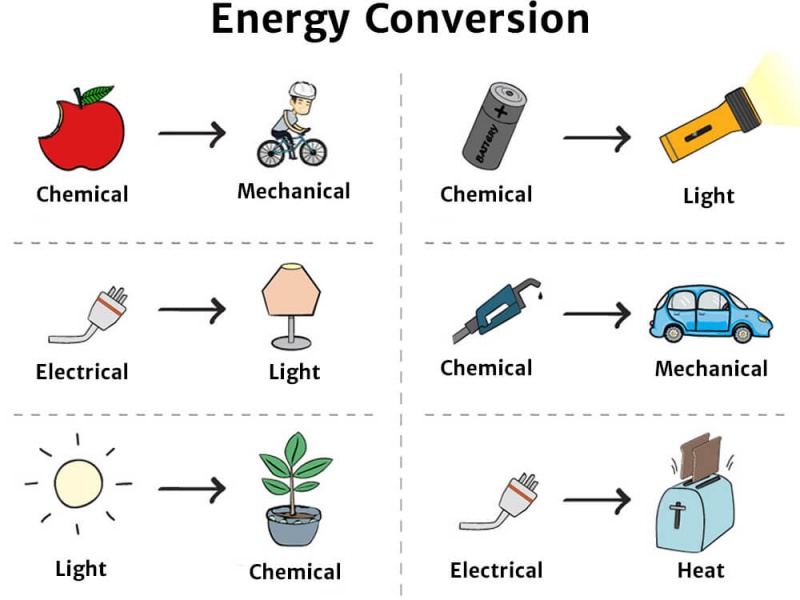
When Energy Is Converted From One Form To Another - The total energy in a system remains constant, although it may be converted from one form to another. Web thermal energy transfers occur in three ways: Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another. Web when energy is converted from one form to another, some portion of the energy produced is lost as thermal energy (heat) that cannot be used for work. Some of these energy converters are quite simple. If a metal spoon is placed in a pot of boiling water, even the end not touching the water gets very hot. Therefore, some fraction of the energy output. Web energy conversion, the transformation of energy from forms provided by nature to forms that can be used by humans. Over the centuries a wide array of devices and systems has been developed for this purpose. Lifting an object) or provides heat.
If a metal spoon is placed in a pot of boiling water, even the end not touching the water gets very hot. Web the first law of thermodynamics states that. Mathematically, this is represented as (1) δ u = q + w with δ u is the total change in internal energy of a system, Web the first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. Some of these energy converters are quite simple. This is also known as the law of conservation of energy or the law of energy conversion. Web the first law of thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be created nor destroyed, under any circumstances. This follows the second law of thermodynamics , which notes that it is impossible to convert all of the thermal energy in a system into useful energy; Over the centuries a wide array of devices and systems has been developed for this purpose. Lifting an object) or provides heat.
This is also known as the law of conservation of energy or the law of energy conversion. Lifting an object) or provides heat. This follows the second law of thermodynamics , which notes that it is impossible to convert all of the thermal energy in a system into useful energy; When thermal energy is transferred between neighboring molecules that are in contact with one another, this is called conduction. Over the centuries a wide array of devices and systems has been developed for this purpose. The early windmills, for example, The total energy in a system remains constant, although it may be converted from one form to another. Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another. Web the first law of thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be created nor destroyed, under any circumstances. Therefore, some fraction of the energy output.
Energy and Heat Physical Science For Dummies
Some of these energy converters are quite simple. Web energy can be converted from one form to another. The total energy in a system remains constant, although it may be converted from one form to another. When thermal energy is transferred between neighboring molecules that are in contact with one another, this is called conduction. Through conduction, convection, and radiation.
Check It Out How Wind Energy Is Converted To Electricity DBLDKR
Some of these energy converters are quite simple. The early windmills, for example, The total energy in a system remains constant, although it may be converted from one form to another. Therefore, some fraction of the energy output. Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another.
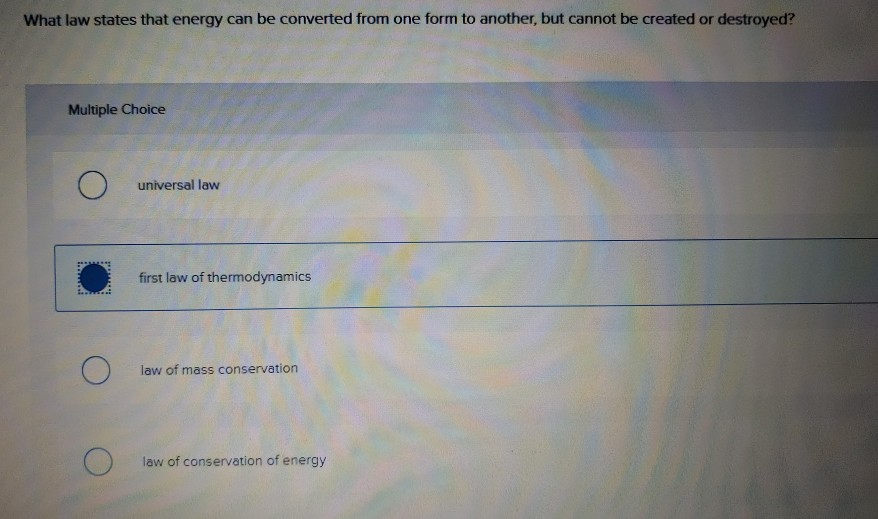
Solved What law states that energy can be converted from one
Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another. This is also known as the law of conservation of energy or the law of energy conversion. Web when energy is converted from one form to another, some portion of the energy produced is lost as thermal energy (heat) that cannot.
Energy Transformation What is Bioenergetics and Free energy
Web thermal energy transfers occur in three ways: Web the first law of thermodynamics states that. Through conduction, convection, and radiation. Web the first law of thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be created nor destroyed, under any circumstances. There are various.
PPT Pearson Prentice Hall Physical Science Concepts in Action
Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another. Some of these energy converters are quite simple. Web energy can be converted from one form to another. Lifting an object) or provides heat. Web energy conversion, the transformation of energy from forms provided by nature to forms that can be.
Albert Einstein Quote “Energy cannot be created or destroyed, it can
Through conduction, convection, and radiation. Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another. Web energy conversion, the transformation of energy from forms provided by nature to forms that can be used by humans. Mathematically, this is represented as (1) δ u = q + w with δ u is.
Energy Conversion Definition and Examples Being Intelligent
Web the first law of thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be created nor destroyed, under any circumstances. Gasoline (chemical) is put into our cars, and with the help of electrical energy from a battery, provides mechanical (kinetic) energy. Web when energy.
Energy Can Be Converted From One Form to Another
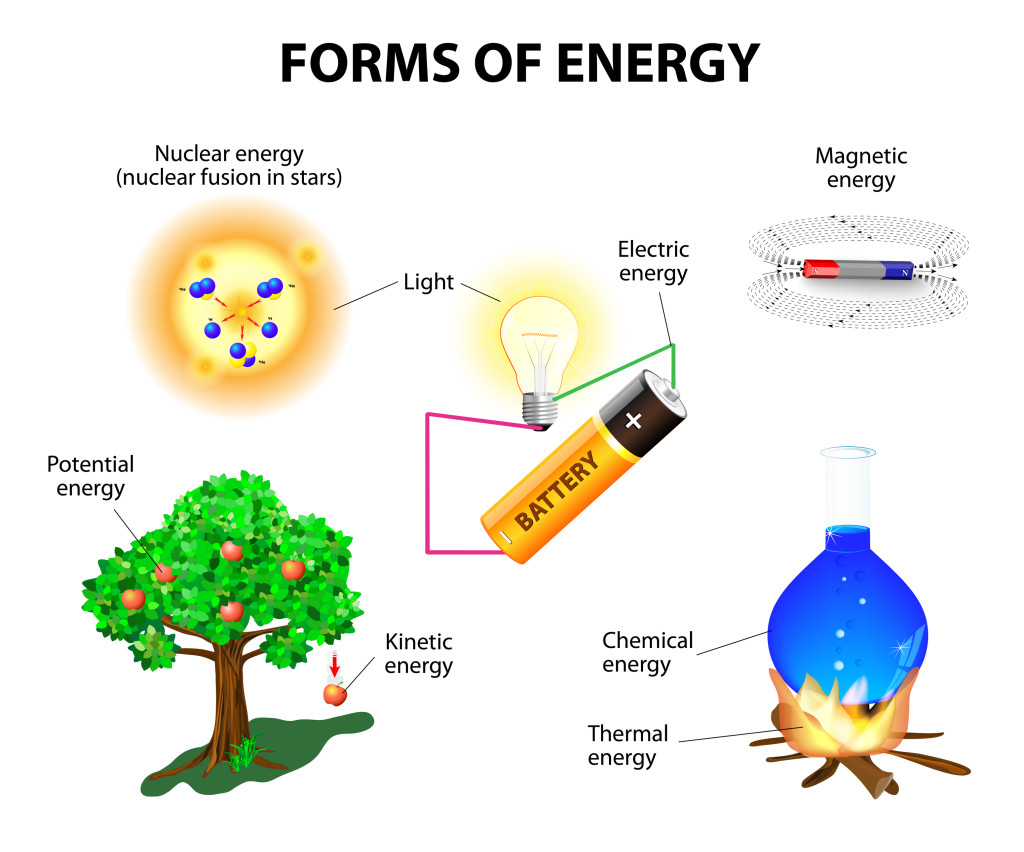
Energy can neither be created nor destroyed, it can only be transformed from one form to another. Web energy can be converted from one form to another. In physics, energy is a quantity that provides the capacity to perform work or moving (e.g. The early windmills, for example, Web energy conversion, the transformation of energy from forms provided by nature.
Forms of Energy
Over the centuries a wide array of devices and systems has been developed for this purpose. The total energy in a system remains constant, although it may be converted from one form to another. Web energy can be converted from one form to another. This is also known as the law of conservation of energy or the law of energy.
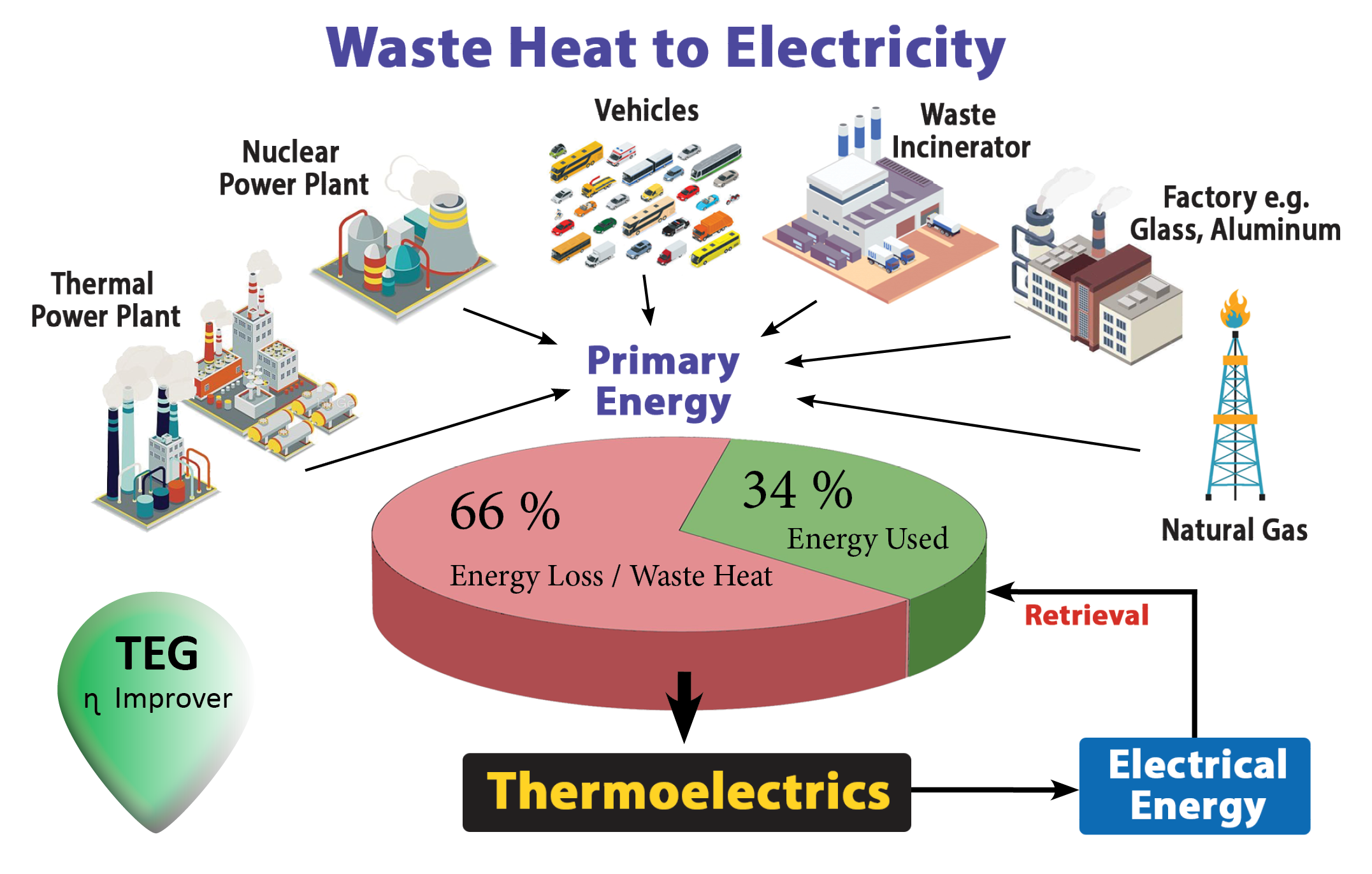
Industry fabrication power conversion technology
Therefore, some fraction of the energy output. Gasoline (chemical) is put into our cars, and with the help of electrical energy from a battery, provides mechanical (kinetic) energy. Over the centuries a wide array of devices and systems has been developed for this purpose. The early windmills, for example, Through conduction, convection, and radiation.
When Thermal Energy Is Transferred Between Neighboring Molecules That Are In Contact With One Another, This Is Called Conduction.
Web thermal energy transfers occur in three ways: There are various types and forms of. Web energy can be converted from one form to another. If a metal spoon is placed in a pot of boiling water, even the end not touching the water gets very hot.
Gasoline (Chemical) Is Put Into Our Cars, And With The Help Of Electrical Energy From A Battery, Provides Mechanical (Kinetic) Energy.
Mathematically, this is represented as (1) δ u = q + w with δ u is the total change in internal energy of a system, Therefore, some fraction of the energy output. Lifting an object) or provides heat. This is also known as the law of conservation of energy or the law of energy conversion.
Web The First Law Of Thermodynamics States That.
Web when energy is converted from one form to another, some portion of the energy produced is lost as thermal energy (heat) that cannot be used for work. Through conduction, convection, and radiation. Some of these energy converters are quite simple. The total energy in a system remains constant, although it may be converted from one form to another.
This Follows The Second Law Of Thermodynamics , Which Notes That It Is Impossible To Convert All Of The Thermal Energy In A System Into Useful Energy;
Web the first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. Over the centuries a wide array of devices and systems has been developed for this purpose. Web energy transformation, also known as energy conversion, is the process of changing energy from one form to another. Energy can neither be created nor destroyed, it can only be transformed from one form to another.