Which Of The Following Would Form An Electrolyte Solution
Which Of The Following Would Form An Electrolyte Solution - Web substances that dissolve in water to yield ions are called electrolytes. Major electrolytes outside the cell. Web this problem has been solved! Holding 100ml of water (ebkare)________________2. Web important electrolytes other than sodium and chloride include potassium, calcium, bicarbonate and phosphate. Web beverages like coconut water and fruit smoothies also provide electrolytes. Web when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Web the current view of electrolyte solutions is that, in water at normal temperatures, the salts of strong acids and strong bases are completely dissociated into ions at all. Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. For example, nacl, hno 3, hclo 3, cacl 2 etc.
Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Web the current view of electrolyte solutions is that, in water at normal temperatures, the salts of strong acids and strong bases are completely dissociated into ions at all. Electrolysis is the decomposition of an electrolyte by an electric current. Group of answer choices sulfuric acid, h2so4 ethanol,. These substances constitute an important class of. Which of the following would form an electrolyte solution when. For example, nacl, hno 3, hclo 3, cacl 2 etc. Web when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Web this problem has been solved! The most important electrolytes found in the highest amounts in the body are sodium,.
Which of the following would form an electrolyte solution when. These substances constitute an important class of. Web when these minerals dissolve in a fluid, they form electrolytes — positive or negative ions in metabolic processes. Web beverages like coconut water and fruit smoothies also provide electrolytes. Web verified answered this week 1 of 3 salt in water would form an electrolyte solution. Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Major electrolytes outside the cell. Group of answer choices sulfuric acid, h2so4 ethanol,. Web which of the following solutes would form electrolyte solutions when dissolved in water?
Solved Classify each of the following solutes as a strong
Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Electrolyte solutions are composed of ions dissolved in water allowing it to conduct. Web the current view of electrolyte solutions is that, in water at normal temperatures, the salts of strong acids and strong bases are completely dissociated into ions at all. Choose.
Question a0b96 + Example
Web verified answered this week 1 of 3 salt in water would form an electrolyte solution. Electrolytes found in your body include: Electrolyte solutions are composed of ions dissolved in water allowing it to conduct. Web the molten or dissolved substance is called the electrolyte. Web which of the following solutes would form electrolyte solutions when dissolved in water?
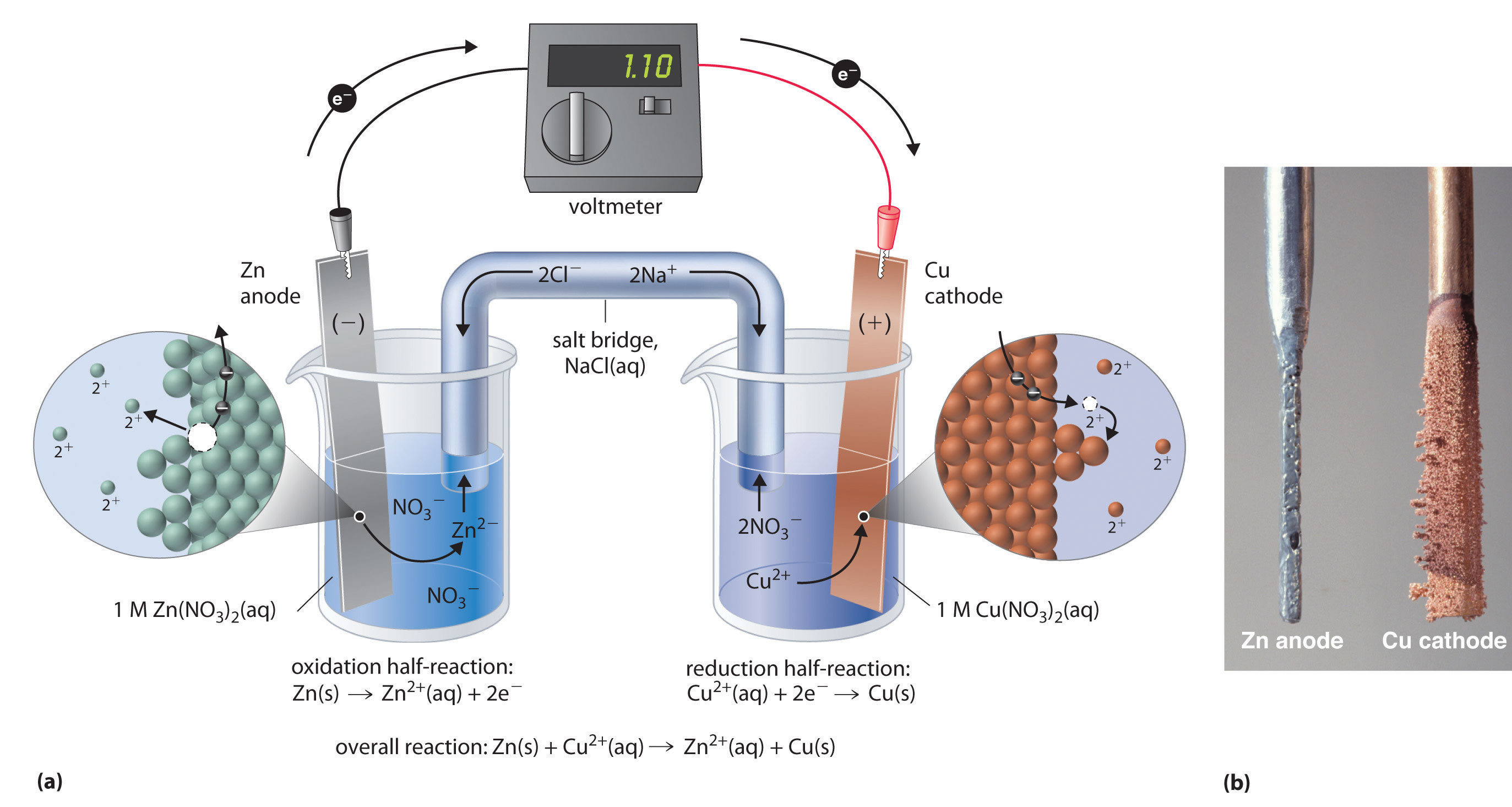
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode
The most important electrolytes found in the highest amounts in the body are sodium,. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids. For example, nacl, hno 3, hclo 3, cacl 2 etc. Which of the following.
Solved 1. Which of the following substances is a strong
Major electrolytes outside the cell. It is used to extract reactive. Web when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Web strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Web the molten or dissolved substance is called the electrolyte.
4.1 Electrolytes Chemistry LibreTexts
Web when these minerals dissolve in a fluid, they form electrolytes — positive or negative ions in metabolic processes. Web this problem has been solved! Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Web important electrolytes other than sodium and chloride include potassium, calcium, bicarbonate and phosphate. The most important electrolytes found in the highest amounts in the body are sodium,.
Electrolytic Cells Video & Lesson Transcript
Web this problem has been solved! Web beverages like coconut water and fruit smoothies also provide electrolytes. Electrolysis is the decomposition of an electrolyte by an electric current. The most important electrolytes found in the highest amounts in the body are sodium,. Web the molten or dissolved substance is called the electrolyte.
Electrolytes Chemistry for Majors
It is used to extract reactive. Web the molten or dissolved substance is called the electrolyte. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Web strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Major electrolytes outside the cell.
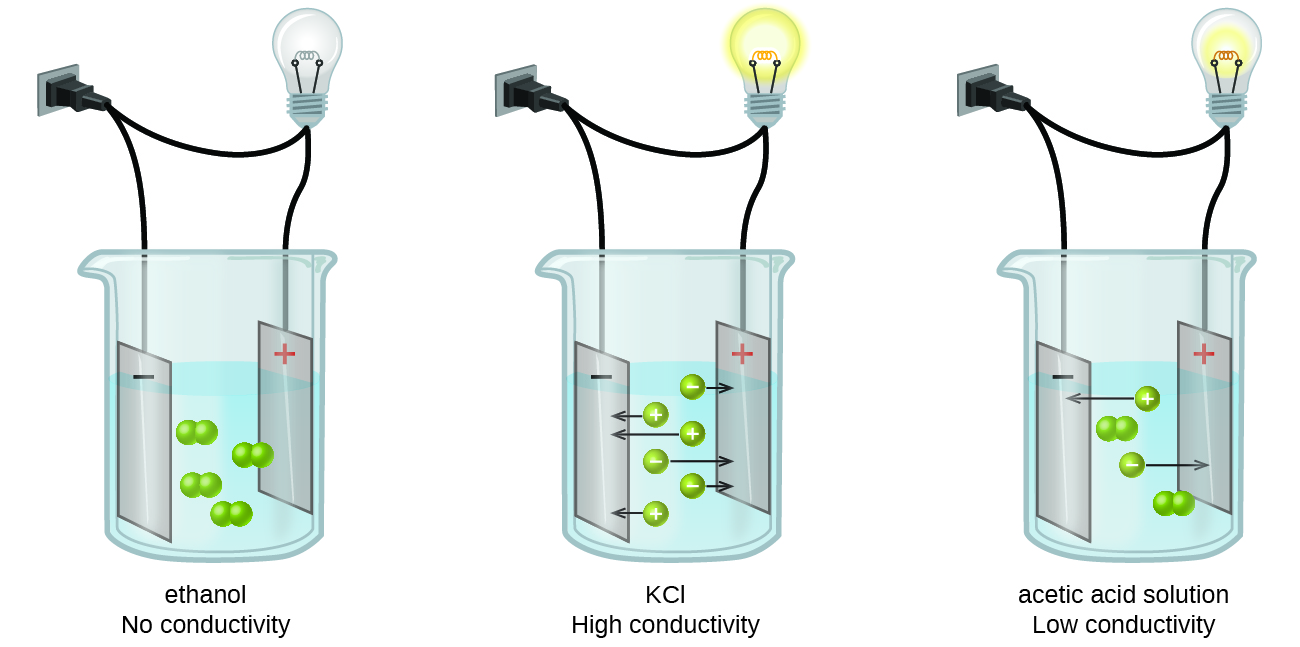
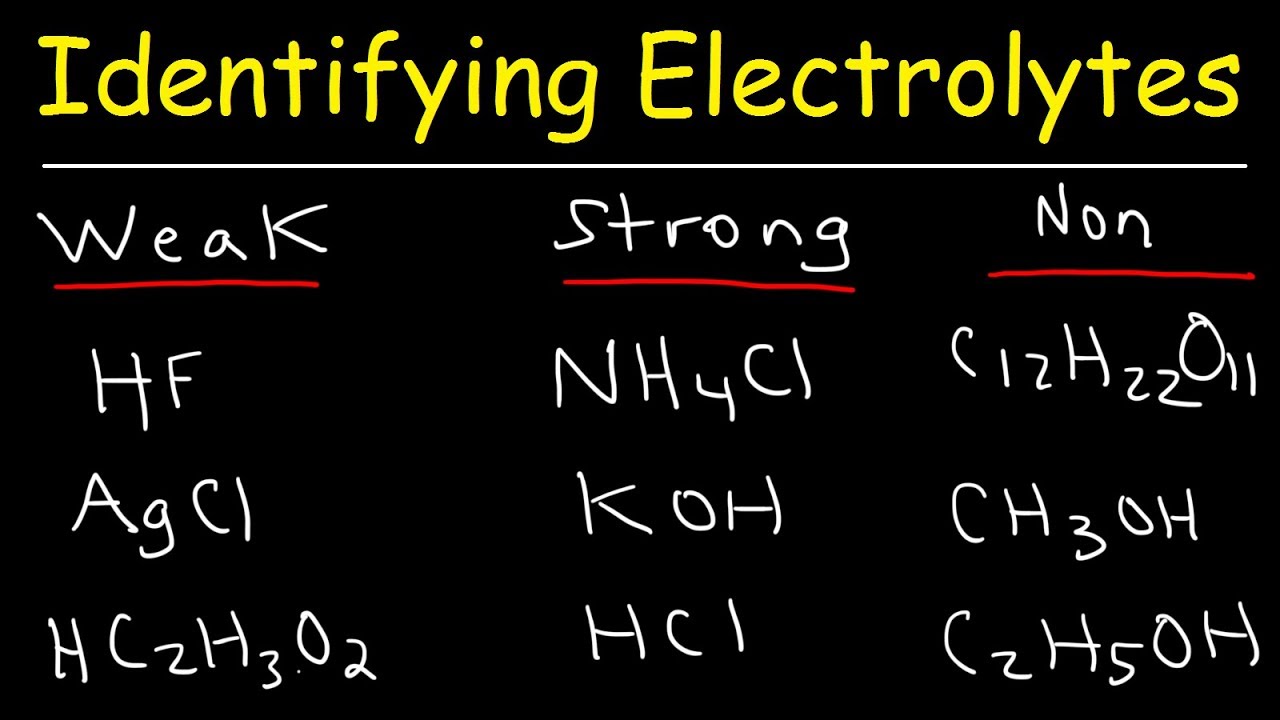
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes
Choose all that are correct. Holding 100ml of water (ebkare)________________2. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers di question 4 0.75 pts which of the following would not form an electrolyte solution when added to water? Web beverages like coconut water and fruit smoothies also.
Daniell Cell Study Material for IIT JEE askIITians
Choose all that are correct. Major electrolytes outside the cell. For example, nacl, hno 3, hclo 3, cacl 2 etc. The most important electrolytes found in the highest amounts in the body are sodium,. Web the molten or dissolved substance is called the electrolyte.
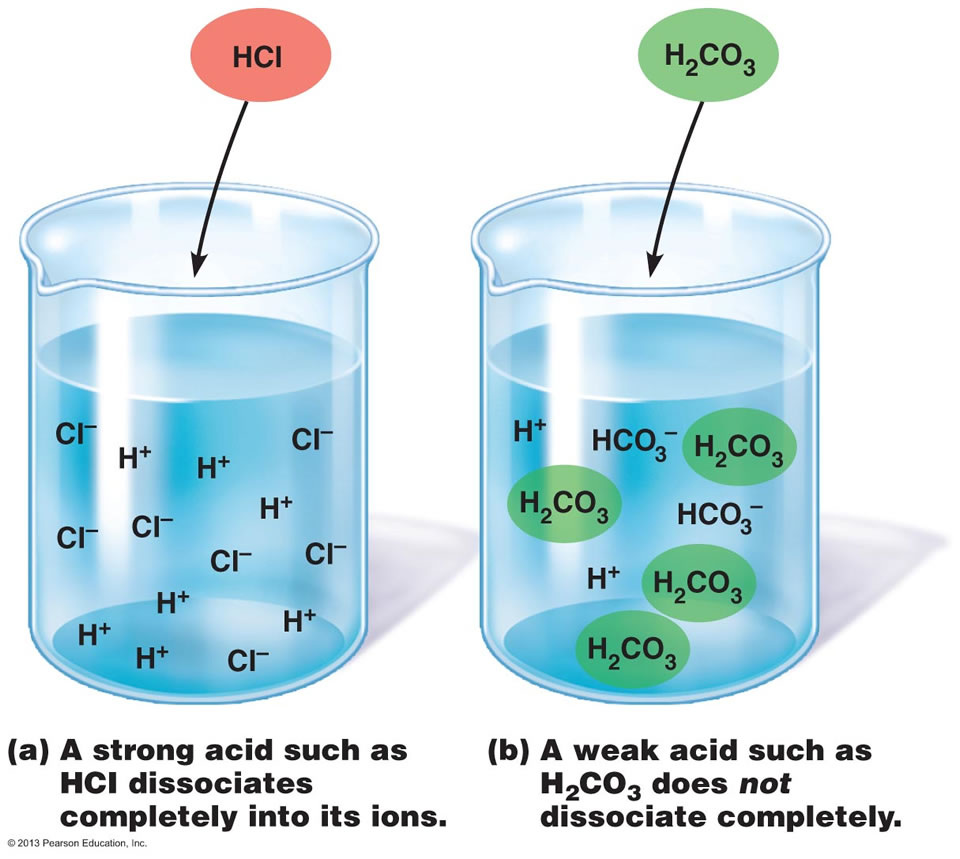
Which Of The Following Diagrams Represent A Weak Acid Dissolved In
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers di question 4 0.75 pts which of the following would not form an electrolyte solution when added to water? Web important electrolytes other than sodium and chloride include potassium, calcium, bicarbonate and phosphate. Web substances that dissolve in.
Web When These Minerals Dissolve In A Fluid, They Form Electrolytes — Positive Or Negative Ions In Metabolic Processes.
Web when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Holding 100ml of water (ebkare)________________2. Web substances that dissolve in water to yield ions are called electrolytes. Web strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution.
Web Which Of The Following Solutes Would Form Electrolyte Solutions When Dissolved In Water?
Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which of the following would form an electrolyte solution when. Web when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution.
Web The Molten Or Dissolved Substance Is Called The Electrolyte.
These substances constitute an important class of. Choose all that are correct. Major electrolytes outside the cell. Group of answer choices sulfuric acid, h2so4 ethanol,.
Web Verified Answered This Week 1 Of 3 Salt In Water Would Form An Electrolyte Solution.
Web the current view of electrolyte solutions is that, in water at normal temperatures, the salts of strong acids and strong bases are completely dissociated into ions at all. Web important electrolytes other than sodium and chloride include potassium, calcium, bicarbonate and phosphate. Electrolyte solutions are composed of ions dissolved in water allowing it to conduct. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.