Why Are Halogens And Alkali Metals Likely To Form Ions
Why Are Halogens And Alkali Metals Likely To Form Ions - Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain one electron. Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Web when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are known as the alkali metals halides. Web when do chemical bonds form? Web the electrolysis is carried out in an argon atmosphere rather than the nitrogen atmosphere typically used for substances that are highly reactive with o 2 and water because li. Web so halogens tend to gain one electron to form a monovalent anion or negatively charged ion and achieve the stable noble gas configuration. Web atomic and physical properties of halogens. Halogen group (group 17) trends. Most binary halides are ionic. So, as you know, alkaline metals are group one, and they all have.
Web when do chemical bonds form? Web the binary compounds of a metal with the halogens are the halides. Web the electrolysis is carried out in an argon atmosphere rather than the nitrogen atmosphere typically used for substances that are highly reactive with o 2 and water because li. Web concept explainers question why are halogens and alkali metals likely to form ions? The alkali metals themselves react. First, there’s a ton of metals. Web halogens such as chlorine, bromine and iodine have properties that enable them to react with other elements to form important salts such as sodium chloride, also. The alkali metals react with the nonmetals in group viia (f 2, cl 2, br 2, i 2, and at 2) to form ionic compounds or salts. Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. They have a low ionization energy, due to them.
Web halogens need to gain only one electron and alkali metals need to lose one electron in order to reach the nearest noble gas configuration and become more stable. When they share or transfer electrons. The halogens are located on the left of the noble gases on the periodic table. Web the electrolysis is carried out in an argon atmosphere rather than the nitrogen atmosphere typically used for substances that are highly reactive with o 2 and water because li. Web okay, so why are hella jin's and alkali metals likely the form ions? Most binary halides are ionic. Web halogens such as chlorine, bromine and iodine have properties that enable them to react with other elements to form important salts such as sodium chloride, also. However, mercury, the elements of group 13 with oxidation states of 3+, tin (iv),. Web concept explainers question why are halogens and alkali metals likely to form ions? Due to the fact that.
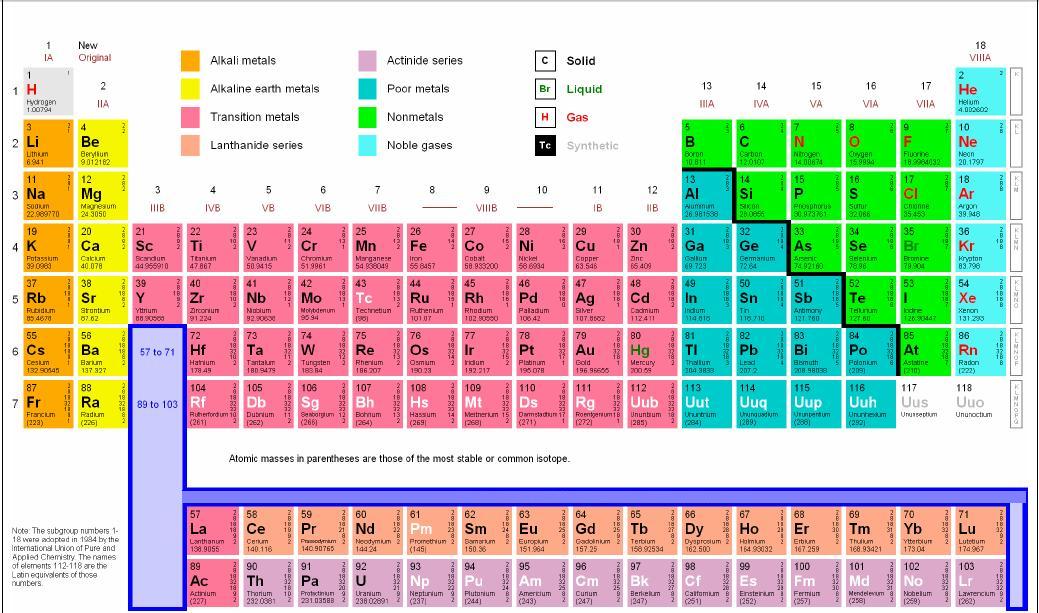
Periodic Table Alkali Metals Halogens Noble Gases Periodic Table Timeline
The halogens are located on the left of the noble gases on the periodic table. Web in the modern iupac nomenclature, this group is known as group (xvii) or group (vii). Halogen group (group 17) trends. Well, the answer lies in the balance show. Web concept explainers question why are halogens and alkali metals likely to form ions?
WJEC Chemistry C2 online revision Reactions of alkali metals and halogens
Web so halogens tend to gain one electron to form a monovalent anion or negatively charged ion and achieve the stable noble gas configuration. Expert solution step by step solved in 2 steps see solution check. Web reactions of alkali metals with group viia. Web halogens such as chlorine, bromine and iodine have properties that enable them to react with.
Solved 53.9 Resources Hint СІ Question 12 of 22 > Metals
Less because i’m putting a couple things i know together to get the explanation. Web concept explainers question why are halogens and alkali metals likely to form ions? Web halogens such as chlorine, bromine and iodine have properties that enable them to react with other elements to form important salts such as sodium chloride, also. [4] the word halogen means.
Solved E) All of the halogens have equal strength as
The alkali metals react with the nonmetals in group viia (f 2, cl 2, br 2, i 2, and at 2) to form ionic compounds or salts. Web in the modern iupac nomenclature, this group is known as group (xvii) or group (vii). Well, the answer lies in the balance show. Web reactions of alkali metals with group viia. However,.
Why Halogens Are So Reactive slidesharedocs
Most binary halides are ionic. Web the binary compounds of a metal with the halogens are the halides. [4] the word halogen means salt former or salt maker. Web when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are known as the alkali metals halides. However, mercury, the elements.
Why are halogens and alkali metals likely to form ions quizlet? Book Vea
Web the electrolysis is carried out in an argon atmosphere rather than the nitrogen atmosphere typically used for substances that are highly reactive with o 2 and water because li. Expert solution step by step solved in 2 steps see solution check. Due to the fact that. Web atomic and physical properties of halogens. The halogens are located on the.
alkali metal Definition, Properties, & Facts Britannica
Web most common nonmetallic substances such as halogens, halogen acids, sulfur, and phosphorus react with the alkali metals. Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain one electron. The alkali metals themselves react. Web they react aggressively with the halogens to form the.
Why are halogens and alkali metals likely to form ions quizlet? Book Vea
Halogen group (group 17) trends. So, as you know, alkaline metals are group one, and they all have. Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain one electron. Web so halogens tend to gain one electron to form a monovalent anion or negatively.
Solved 141 26. Write down two elements that are members of
Less because i’m putting a couple things i know together to get the explanation. The halogens are located on the left of the noble gases on the periodic table. When they share or transfer electrons. Web the binary compounds of a metal with the halogens are the halides. Halogen group (group 17) trends.
Malik Xufyan Only Chemistry Discussion Q. No. 34 why Alkali Metals
The alkali metals themselves react. Web okay, so why are hella jin's and alkali metals likely the form ions? However, mercury, the elements of group 13 with oxidation states of 3+, tin (iv),. [4] the word halogen means salt former or salt maker. The alkali metals react with the nonmetals in group viia (f 2, cl 2, br 2, i.
When They Share Or Transfer Electrons.
Web the electrolysis is carried out in an argon atmosphere rather than the nitrogen atmosphere typically used for substances that are highly reactive with o 2 and water because li. Less because i’m putting a couple things i know together to get the explanation. [4] the word halogen means salt former or salt maker. Web the binary compounds of a metal with the halogens are the halides.
So, As You Know, Alkaline Metals Are Group One, And They All Have.
Due to the fact that. Web okay, so why are hella jin's and alkali metals likely the form ions? Web when do chemical bonds form? Why are halogens and alkali metals likely to form ions?
The Alkali Metals React With The Nonmetals In Group Viia (F 2, Cl 2, Br 2, I 2, And At 2) To Form Ionic Compounds Or Salts.
Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Web when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are known as the alkali metals halides. Web reactions of alkali metals with group viia. They have a low ionization energy, due to them.
Well, The Answer Lies In The Balance Show.
Web halogens such as chlorine, bromine and iodine have properties that enable them to react with other elements to form important salts such as sodium chloride, also. Web most common nonmetallic substances such as halogens, halogen acids, sulfur, and phosphorus react with the alkali metals. The halogens are located on the left of the noble gases on the periodic table. Web they react aggressively with the halogens to form the alkali metal halides, which are white ionic crystalline compounds that are all soluble in water except lithium fluoride (li f).