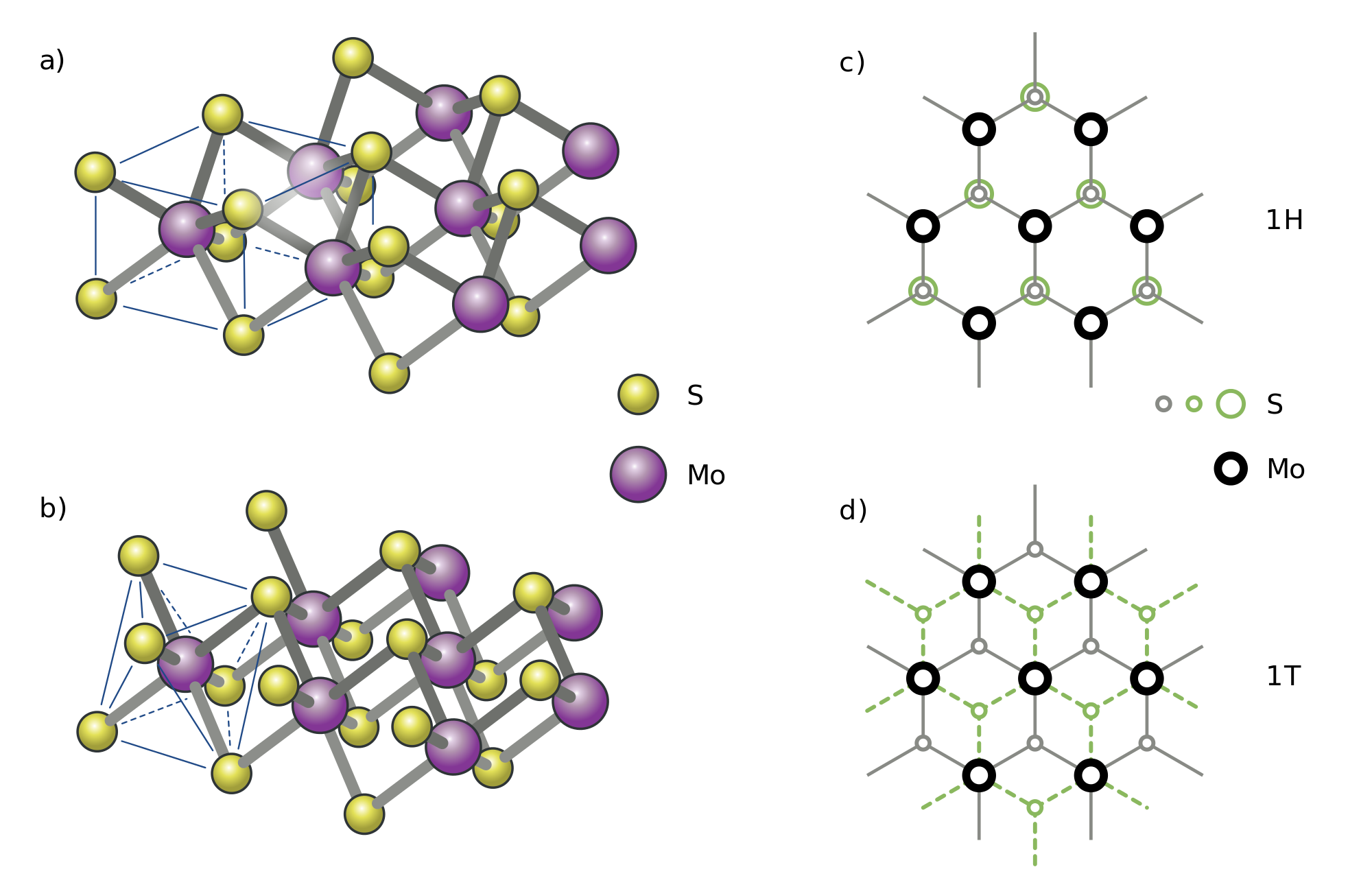
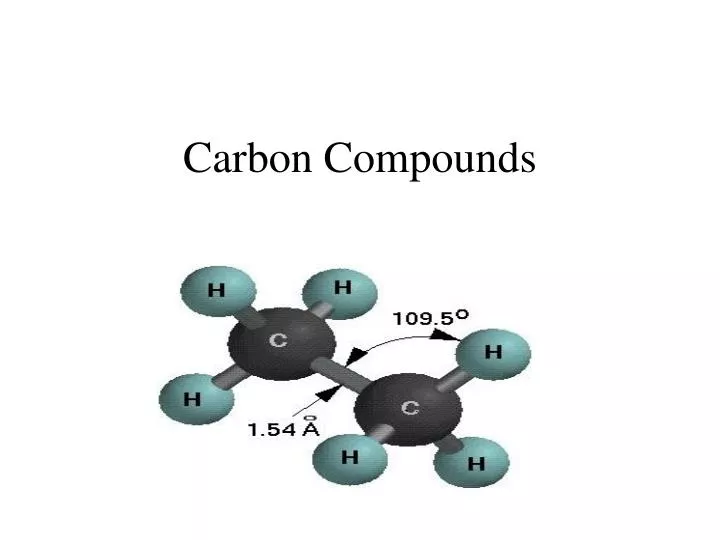
Why Can Carbon Form A Variety Of Organic Compounds
Why Can Carbon Form A Variety Of Organic Compounds - Why don’t we instead use, say, oxygen for the same purpose? Carbon dioxide is considered by chemists as inorganic, along with carbon monoxide, carbonates and bicarbonates. The study of the properties, reactions, and syntheses. Each carbon atom ionically bonds with atoms of carbon, hydrogen,. Web which statement best describes why carbon can form a wide variety of organic compounds? Inorganic compounds there is a rich variety of carbon. There are more carbon compounds than for any other. Web organic chemistry, then, is the study of the compounds of carbon excluding the oxides of carbon and the inorganic metal compounds known as bicarbonates,. Web carbon, chemical element that forms more compounds than all the other elements combined. Web by definition, all organic molecule contain carbon.
Web so it is inorganic. Inorganic compounds there is a rich variety of carbon. Web carbon, chemical element that forms more compounds than all the other elements combined. Why don’t we instead use, say, oxygen for the same purpose? The simplest carbon molecule is methane (ch 4 ), depicted here. Web due to carbon's ability to catenate (form chains with other carbon atoms ), millions of organic compounds are known. Web why is carbon so popular for making molecular backbones? Web carbon compounds are chemical substances that contain carbon atoms bonded to any other element. Web the possibilities for diversity are increased by the presence of atoms other than carbon in organic compounds, especially hydrogen (h), oxygen (o), nitrogen (n), halogens. The study of the properties, reactions, and syntheses.
Carbon can form four covalent bonds to create an organic molecule. Web by definition, all organic molecule contain carbon. There are more carbon compounds than for any other. Web which statement best describes why carbon can form a wide variety of organic compounds? Web organic chemistry, then, is the study of the compounds of carbon excluding the oxides of carbon and the inorganic metal compounds known as bicarbonates,. Web the possibilities for diversity are increased by the presence of atoms other than carbon in organic compounds, especially hydrogen (h), oxygen (o), nitrogen (n), halogens. Carbon is widely distributed in coal and in the compounds that. The reason for that is a little trickier to answer, but basically comes down to carbon having a valence of four (ie. Web due to carbon's ability to catenate (form chains with other carbon atoms ), millions of organic compounds are known. Web so it is inorganic.
PPT Organic Compounds PowerPoint Presentation ID2985134
Web which statement best describes why carbon can form a wide variety of organic compounds? Web carbon compounds are chemical substances that contain carbon atoms bonded to any other element. Carbon dioxide is considered by chemists as inorganic, along with carbon monoxide, carbonates and bicarbonates. Web by definition, all organic molecule contain carbon. The simplest carbon molecule is methane (ch.
Carbon and its Compound Class 10 Notes Science Chapter 4, Explanation
The reason for that is a little trickier to answer, but basically comes down to carbon having a valence of four (ie. Carbon dioxide is considered by chemists as inorganic, along with carbon monoxide, carbonates and bicarbonates. Carbon can form four covalent bonds to create an organic molecule. Each carbon atom ionically bonds with atoms of carbon, hydrogen,. Web why.
PPT Properties of Carbon Element PowerPoint Presentation, free
Inorganic compounds there is a rich variety of carbon. Web carbon, chemical element that forms more compounds than all the other elements combined. Carbon dioxide is considered by chemists as inorganic, along with carbon monoxide, carbonates and bicarbonates. Each carbon atom ionically bonds with atoms of carbon, hydrogen,. Web carbon compounds are chemical substances that contain carbon atoms bonded to.
PPT Properties of Carbon Element PowerPoint Presentation, free
The simplest carbon molecule is methane (ch 4 ), depicted here. Web the possibilities for diversity are increased by the presence of atoms other than carbon in organic compounds, especially hydrogen (h), oxygen (o), nitrogen (n), halogens. Web organic chemistry, then, is the study of the compounds of carbon excluding the oxides of carbon and the inorganic metal compounds known.
Top 50 Questions of Carbon and its Compound Lesson
The study of the properties, reactions, and syntheses. There are more carbon compounds than for any other. Web why is carbon so popular for making molecular backbones? Carbon is widely distributed in coal and in the compounds that. The simplest carbon molecule is methane (ch 4 ), depicted here.
Carbon and Its Compounds Class 10 NCERT Notes Leverage Edu
There are more carbon compounds than for any other. Web so it is inorganic. The study of the properties, reactions, and syntheses. Web carbon compounds are chemical substances that contain carbon atoms bonded to any other element. The simplest carbon molecule is methane (ch 4 ), depicted here.
PPT Properties of Carbon Element PowerPoint Presentation ID4693595
The simplest carbon molecule is methane (ch 4 ), depicted here. Carbon can form four covalent bonds to create an organic molecule. Web carbon compounds are chemical substances that contain carbon atoms bonded to any other element. Web carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on earth,.
PPT Properties of Carbon Element PowerPoint Presentation, free
Web carbon, chemical element that forms more compounds than all the other elements combined. Web carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on earth, enables this. Web due to carbon's ability to catenate (form chains with other carbon atoms ), millions of organic compounds are known. Web.
PPT Carbon Compounds PowerPoint Presentation, free download ID174669
Web why is carbon so popular for making molecular backbones? Web due to carbon's ability to catenate (form chains with other carbon atoms ), millions of organic compounds are known. The simplest carbon molecule is methane (ch 4 ), depicted here. Each carbon atom ionically bonds with atoms of carbon, hydrogen,. Web the possibilities for diversity are increased by the.
Carbon Compounds and Examples
Why don’t we instead use, say, oxygen for the same purpose? Web by definition, all organic molecule contain carbon. Web carbon compounds are chemical substances that contain carbon atoms bonded to any other element. Web organic chemistry, then, is the study of the compounds of carbon excluding the oxides of carbon and the inorganic metal compounds known as bicarbonates,. Carbon.
Carbon Is Widely Distributed In Coal And In The Compounds That.
Carbon can form four covalent bonds to create an organic molecule. Inorganic compounds there is a rich variety of carbon. Each carbon atom ionically bonds with atoms of carbon, hydrogen,. Web with carbon bonded to metals the field of organic chemistry crosses over into organometallic chemistry.
Web Why Is Carbon So Popular For Making Molecular Backbones?
Web carbon, chemical element that forms more compounds than all the other elements combined. The simplest carbon molecule is methane (ch 4 ), depicted here. Web carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on earth, enables this. Web due to carbon's ability to catenate (form chains with other carbon atoms ), millions of organic compounds are known.
The Study Of The Properties, Reactions, And Syntheses.
Web the possibilities for diversity are increased by the presence of atoms other than carbon in organic compounds, especially hydrogen (h), oxygen (o), nitrogen (n), halogens. Web organic chemistry, then, is the study of the compounds of carbon excluding the oxides of carbon and the inorganic metal compounds known as bicarbonates,. Carbon dioxide is considered by chemists as inorganic, along with carbon monoxide, carbonates and bicarbonates. Web so it is inorganic.
Why Don’t We Instead Use, Say, Oxygen For The Same Purpose?
The reason for that is a little trickier to answer, but basically comes down to carbon having a valence of four (ie. Web carbon compounds are chemical substances that contain carbon atoms bonded to any other element. Web which statement best describes why carbon can form a wide variety of organic compounds? There are more carbon compounds than for any other.