Neon Electron Configuration Long Form
Neon Electron Configuration Long Form - Possible oxidation states are 0. Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. Ne (neon) is an element with position number 10 in the periodic table. Web electron configuration of neon is [he] 2s2 2p6. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Web electronic configuration of neon (ne): Web using aufbau principle. ← electronic configurations of elements. The electron configurations and orbital.
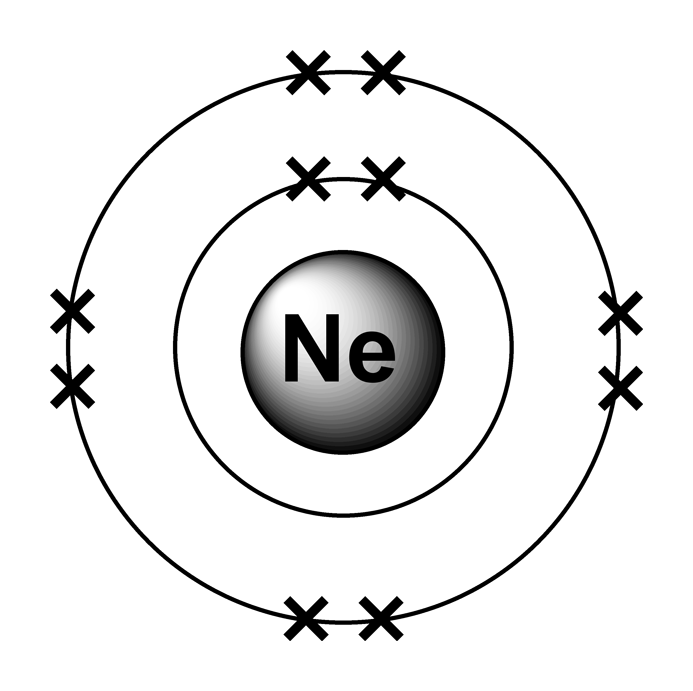
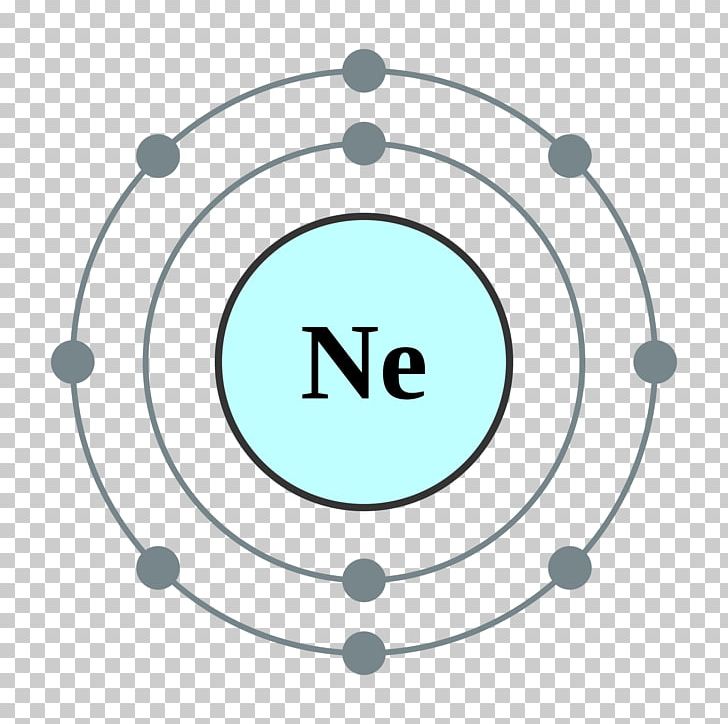
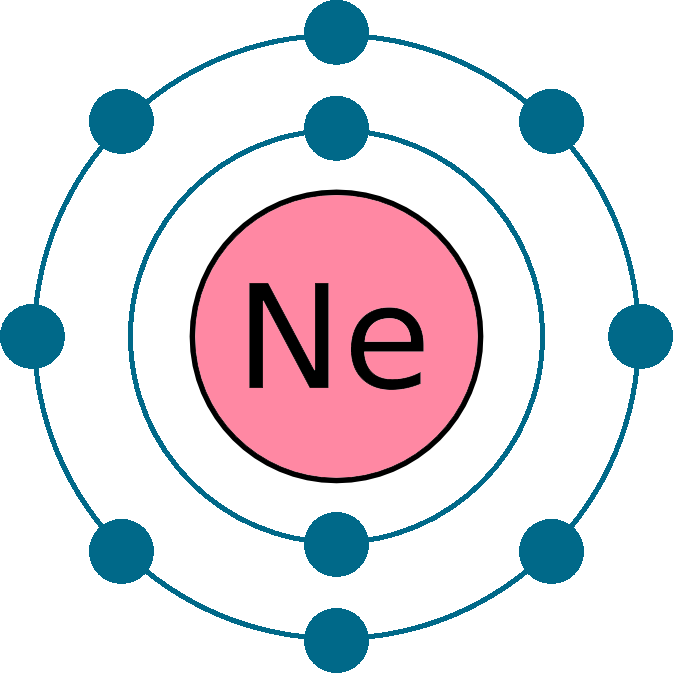
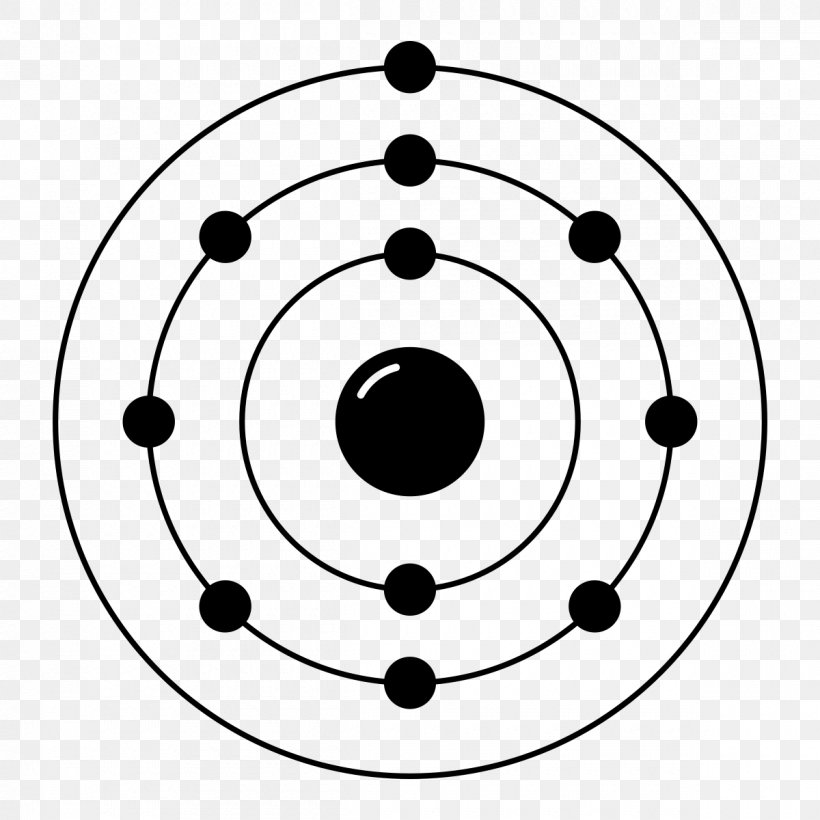
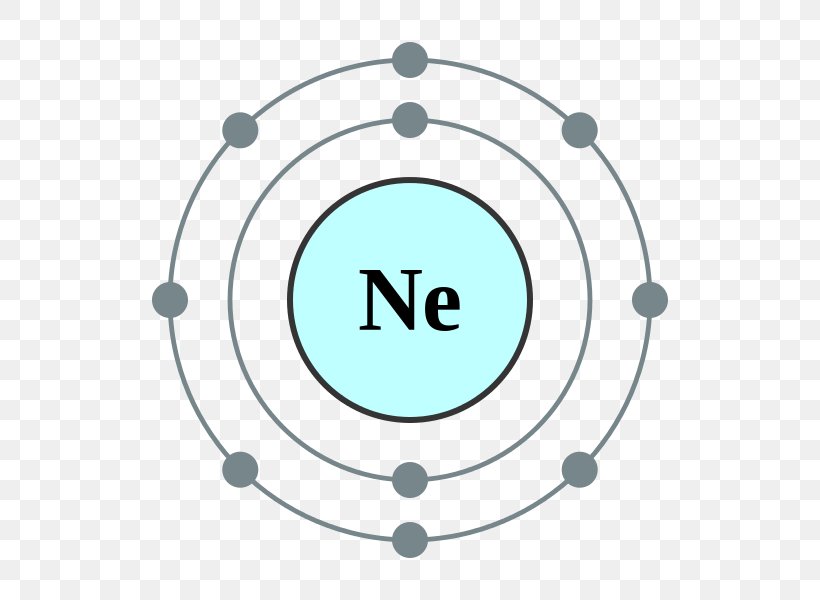
Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web neon electron configuration number. Typical densities of various substances are at. The n=1 shell can only hold 2 electrons, so the remaining 8. For each atom the subshells are given first in concise form, then with all. ← electronic configurations of elements. Possible oxidation states are 0. Web electronic configuration of neon (ne): Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The configuration number of the electron is nothing but the same equation that represents the configuration for the.
The electronic configuration of neon is ne: For each atom the subshells are given first in concise form, then with all. Neon is a very inert element, however, it has been reported to form a compound with fluorine. The n=1 shell can only hold 2 electrons, so the remaining 8. Typical densities of various substances are at. Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. Ne (neon) is an element with position number 10 in the periodic table. Since the atomic number of neon is 10, the total electrons. Located in the ii period. Web electron configuration of neon is [he] 2s2 2p6.
Electron configurations
Web neon electron configuration number. Web electronic configuration of neon (ne): Possible oxidation states are 0. 1s 2 2s 2 2p 6. Web the first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium.
Neon(Ne) electron configuration and orbital diagram
Web neon is the tenth element with a total of 10 electrons. Web using aufbau principle. Neon is a very inert element, however, it has been reported to form a compound with fluorine. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the.
47 A ELECTRON CONFIGURATION OF NEON * ElectronConfiguration
Since 1s can only hold two. For each atom the subshells are given first in concise form, then with all. It is in group 18(noble gases) of the periodic table. Possible oxidation states are 0. Web using aufbau principle.
Neon Electron Configuration Noble Gas Valence Electron Lewis Structure
Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. So, the electron configuration of neon is 1 s 2 2 s 2 2 p 6 now, the neon. Possible oxidation states are 0. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web.
Electron Configuration for Neon (Ne) Full Explanation
Web neon electron configuration number. Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. Density of neon density of neon is 0.0009g/cm3. Since the atomic number of neon is 10, the total electrons. Web the first two electrons in lithium fill the 1 s orbital and have the same.
Neon Element (Ne 10) of Periodic Table Periodic Table FlashCard
Web the first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. 1s 2 2s 2 2p 6. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Neon is a very inert element, however, it has been.
Neon Electron Configuration Bohr Model Electron Shell Noble Gas, PNG
Web neon is the tenth element with a total of 10 electrons. The electron configurations and orbital. For each atom the subshells are given first in concise form, then with all. In order to write the ne electron configuration we first need to know the number of elect show. Web neon has atomic number 10, so a neon atom has.
Electron Configuration Noble Gas Neon Electron Shell, PNG, 600x600px
Web electronic configuration of neon (ne): The remaining electron must occupy the orbital. Web the first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. Since 1s can only hold two. Located in the ii period.
The Compound Neon Electron Configuration Scholars Ark
In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Web neon electron configuration number. Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number.
[5 Steps] Electron Configuration for or of Neon in Just 5 Steps
Web electron configuration of neon is [he] 2s2 2p6. Possible oxidation states are 0. The n=1 shell can only hold 2 electrons, so the remaining 8. Density of neon density of neon is 0.0009g/cm3. Web electronic configuration of neon (ne):
It Is Still Questionable If True Compounds Of Neon Exist, But.
The atomic number of neon represents the total number of electrons of neon. ← electronic configurations of elements. 1s 2 2s 2 2p 6. The electronic configuration of neon is ne:
Web Using Aufbau Principle.
Ne (neon) is an element with position number 10 in the periodic table. Web neon electron configuration number. Located in the ii period. So, the electron configuration of neon is 1 s 2 2 s 2 2 p 6 now, the neon.
Possible Oxidation States Are 0.
Web the complete electron configuration of neon is 1s 2 2s 2 2p 6.neon has 8 valence electrons around the nucleus and the atomic number is 10. Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. For each atom the subshells are given first in concise form, then with all. Since the atomic number of neon is 10, the total electrons.
Web The First Two Electrons In Lithium Fill The 1 S Orbital And Have The Same Sets Of Four Quantum Numbers As The Two Electrons In Helium.
Since 1s can only hold two. Typical densities of various substances are at. Web neon is the tenth element with a total of 10 electrons. It is in group 18(noble gases) of the periodic table.




![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)