What Two Ions Form When Water Dissociates
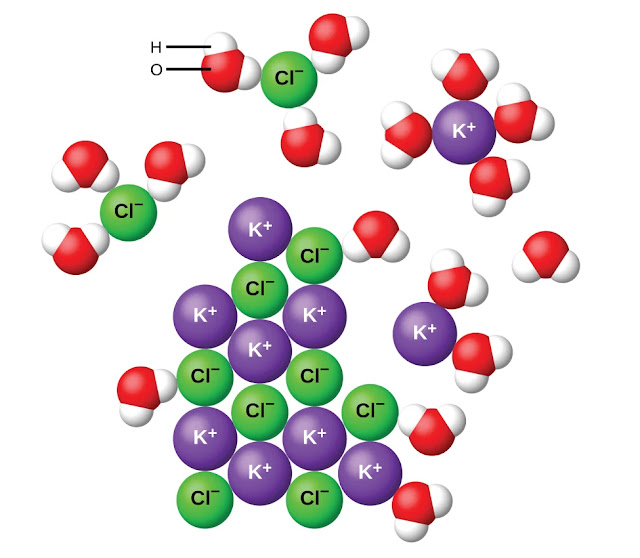
What Two Ions Form When Water Dissociates - Owing to the overwhelming excess of. Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water. Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,. Web docbsullivan water properties, acids, bases, & buffers study play polar molecule a molecule w/ opposite charges on opposite ends why is water a polar molecule? Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity. Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. And arrhenius definition states ensure an acid produces h+ in resolution and a base. Acids produce hydrogen ions due to dissociation. Web for instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (h +). Web an ionic crystal lattice breaks apart when it is dissolved in water.
Web docbsullivan water properties, acids, bases, & buffers study play polar molecule a molecule w/ opposite charges on opposite ends why is water a polar molecule? And arrhenius definition states ensure an acid produces h+ in resolution and a base. Web there is, however, a constant change; Owing to the overwhelming excess of. Web study with quizlet and memorize flashcards containing terms like define polar molecule, why is water considered polar?, explain hydrogen bonding in terms of water. Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water. Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. Web what ion forms when water dissociates? The following equation describes the process in. Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity.
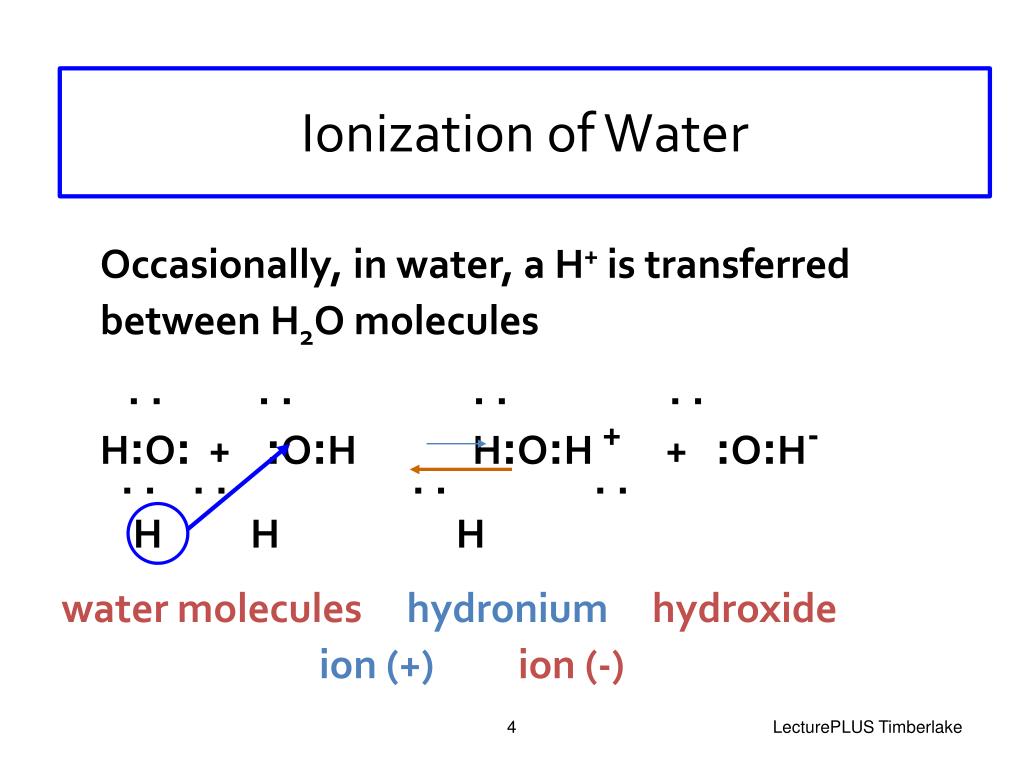
As one hydrogen ion reattaches to a black ion to form a water molecule, another soak molecule dissociates to replace the hydrogen ion. Web reactions in aqueous solutions. Acids produce hydrogen ions due to dissociation. Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,. For example, sodium chloride (nacl). Web study with quizlet and memorize flashcards containing terms like define polar molecule, why is water considered polar?, explain hydrogen bonding in terms of water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. When a molecular compound undergoes dissociation into ions, the. Web what does 2 ions are formed when water dissociates?
MakeTheBrainHappy The Lewis Dot Structure for KCl
Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,. Web about one in half a billion water molecules act as einen.
What is the pH of a .001 M solution of HCl? Socratic
Web there is, however, a constant change; Web an ionic crystal lattice breaks apart when it is dissolved in water. And arrhenius definition states ensure an acid produces h+ in resolution and a base. As one hydrogen ion reattaches to a black ion to form a water molecule, another soak molecule dissociates to replace the hydrogen ion. Web pure water.
PPT Dissociation of Water PowerPoint Presentation, free download ID
Web what ion forms when water dissociates? For example, sodium chloride (nacl). Web an ionic crystal lattice breaks apart when it is dissolved in water. Web there is, however, a constant change; Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water.
Write The Chemical Formulas Of Two Ions When Table Salt Dissolved In
Owing to the overwhelming excess of. Web what does 2 ions are formed when water dissociates? Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity. Web about one in half a billion water molecules act as einen acid by give a molecule to other surface molecule that acts as.
PPT Explaining the Properties of Acids & Bases PowerPoint
Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity. Acids produce hydrogen ions due to dissociation. For example, sodium chloride (nacl). Web study with quizlet and memorize flashcards containing terms like define polar molecule, why is water considered polar?, explain hydrogen bonding in terms of water. Web what does.
What Happens When an Acid & a Base Are Combined? Sciencing
Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. Web what ion forms when water dissociates? Web reactions in aqueous solutions. Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,..
Solved Benzoic acid dissociates in water to form hydrogen
Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. For example, sodium chloride (nacl). Web for instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (h +). Web the reaction in which water breaks into hydrogen and.
6. (4) Neither C,H,OH nor water Consider Physical Chemistry
Web what ion forms when water dissociates? Web study with quizlet and memorize flashcards containing terms like define polar molecule, why is water considered polar?, explain hydrogen bonding in terms of water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Web on are three major classifications of substances known as acids or bases. Web.
What Is pH? — Definition & Overview Expii
Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water. Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,. Owing to the overwhelming excess.
Solutions
Web on are three major classifications of substances known as acids or bases. Web what does 2 ions are formed when water dissociates? Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water. Web the reaction in which water breaks into hydrogen and hydroxide ions is a.
Web The Reaction In Which Water Breaks Into Hydrogen And Hydroxide Ions Is A Dissociation Reaction.
Web reactions in aqueous solutions. Web an ionic crystal lattice breaks apart when it is dissolved in water. Web on are three major classifications of substances known as acids or bases. Web for instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (h +).
Web Docbsullivan Water Properties, Acids, Bases, & Buffers Study Play Polar Molecule A Molecule W/ Opposite Charges On Opposite Ends Why Is Water A Polar Molecule?
What ions form when ca (no3)2 disassociates? Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Web what does 2 ions are formed when water dissociates? Web about one in half a billion water molecules act as einen acid by give a molecule to other surface molecule that acts as a base, producing hydroxide ion and.
Web What Ion Forms When Water Dissociates?
The following equation describes the process in. Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water. For example, sodium chloride (nacl). Web there is, however, a constant change;
Owing To The Overwhelming Excess Of.
Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,. As one hydrogen ion reattaches to a black ion to form a water molecule, another soak molecule dissociates to replace the hydrogen ion. And arrhenius definition states ensure an acid produces h+ in resolution and a base. Acids produce hydrogen ions due to dissociation.