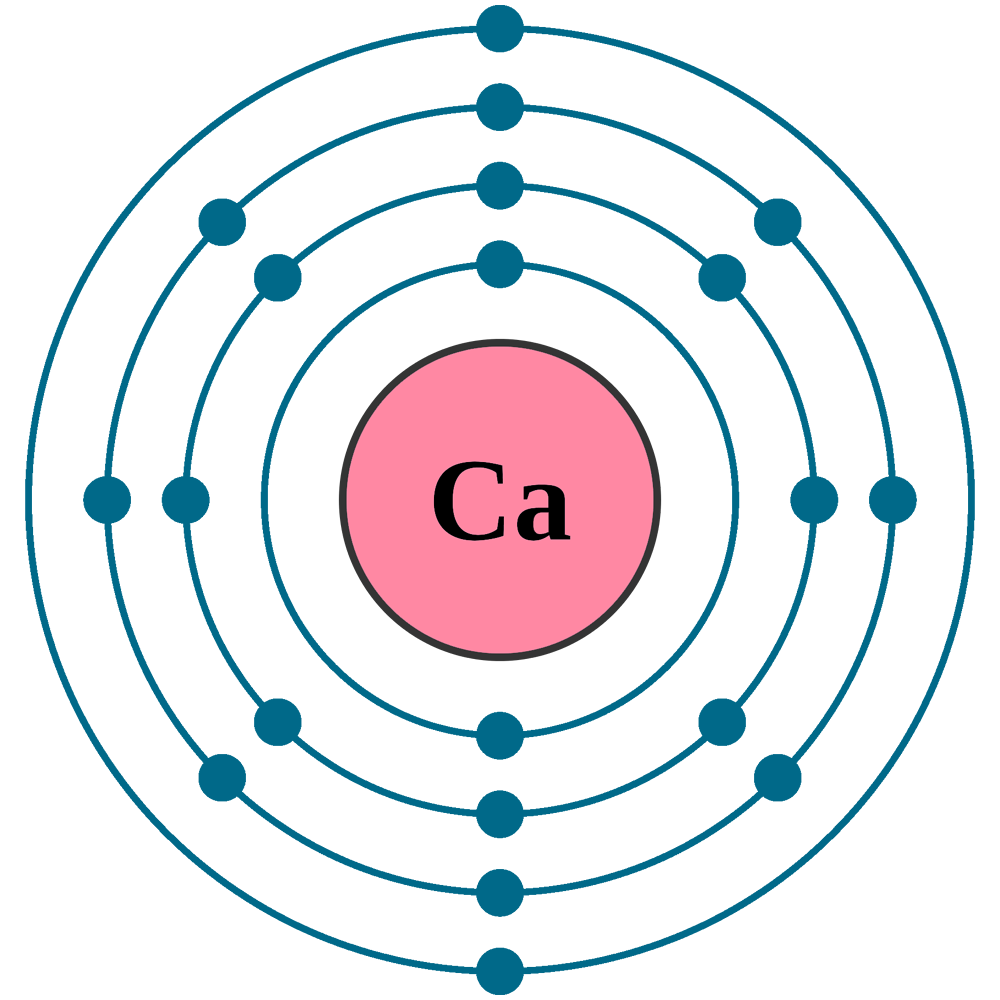
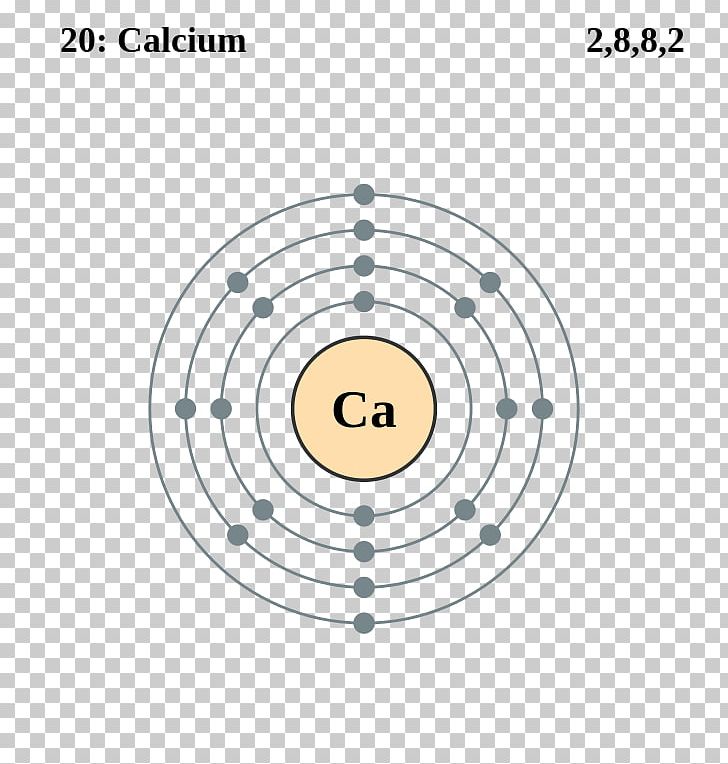
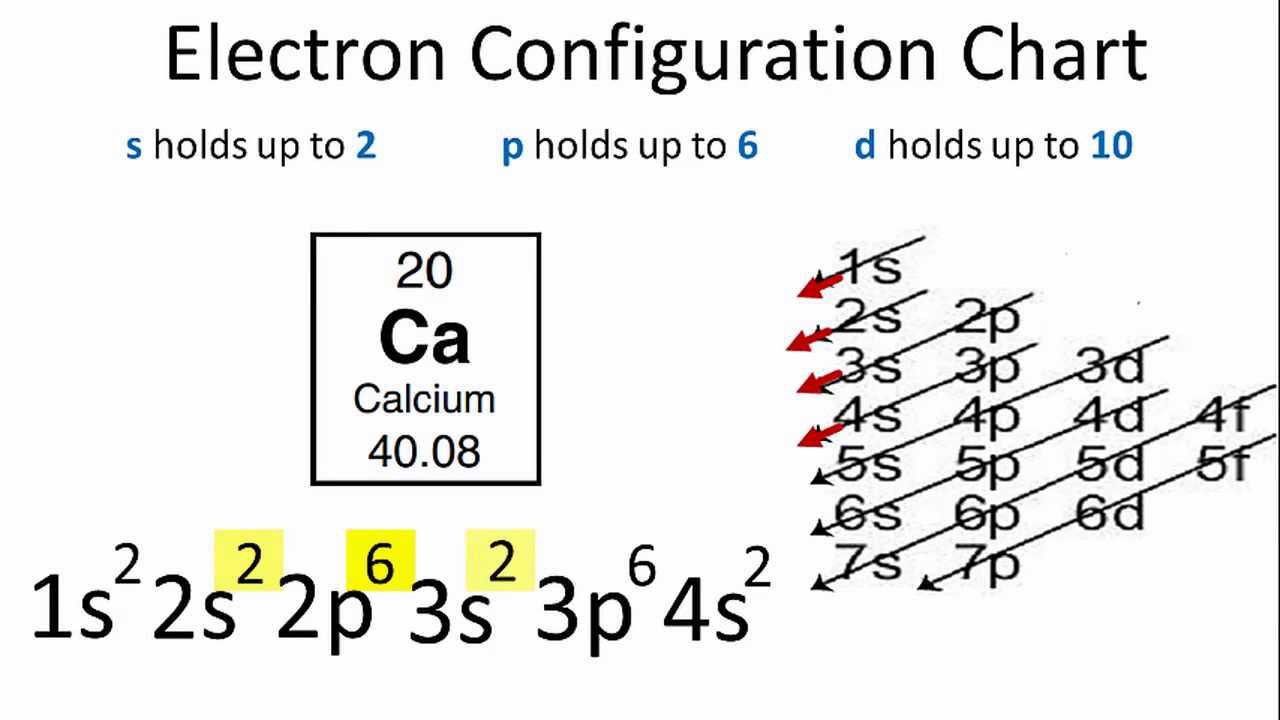
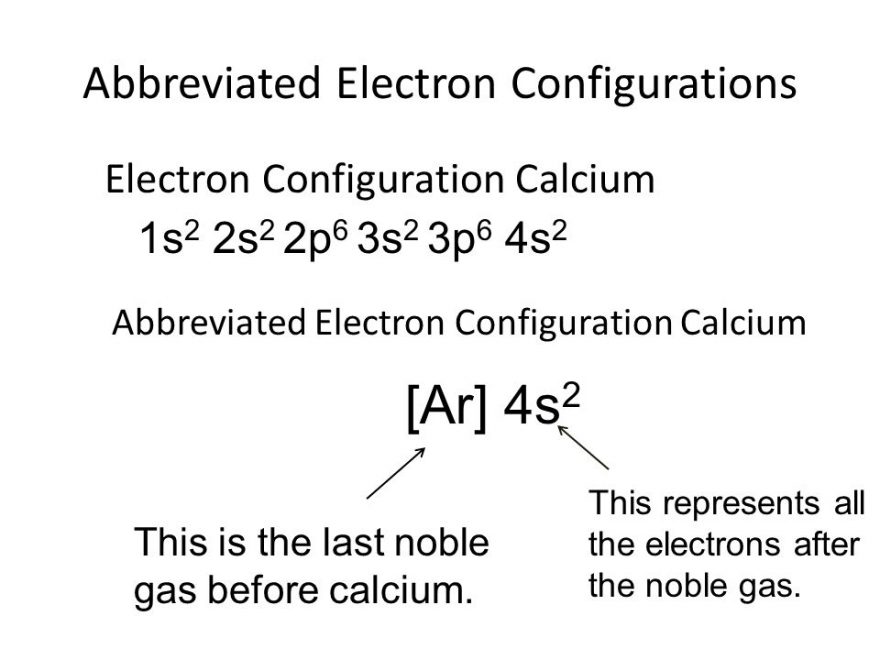
Calcium Electron Configuration Long Form
Calcium Electron Configuration Long Form - Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Electron configuration of oxygen (o) [he] 2s 2 2p 4: According to this configuration, how many electrons are found on the valence level? The electron configuration of calcium is given as 1s22s22p63s23p64s2. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web the electron configuration of calcium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 , if the electron arrangement is through orbitals. Web the electron configuration of calcium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 calcium is metal because it is in group 2 which makes it an alkaline earth metal and a powerful reducing agent. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si) phosphorus (p) sulfur (s) chlorine (cl) argon (ar) potassium (k).
Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si) phosphorus (p) sulfur (s) chlorine (cl) argon (ar) potassium (k). Web electron configuration of carbon (c) [he] 2s 2 2p 2: The electron configuration of calcium is. Web the electronic configuration of calcium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2. Write electronic configuration for cu, cu2+, zn2+, cr3+. Simply use this information to obtain its electronic configuration. Calcium isotopes are 40 ca, 42 ca, 43 ca, 44 ca, 46 ca, and 48 ca. The next six electrons will go in the 2p orbital. According to this configuration, how many electrons are found on the valence level? Radium pls write in full withoutbridging with the noble gases.
Web calcium has an atomic number of 20. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Electron configurations of elements beyond hassium (element 108) have never been measured; Web this video help for you.how to write the electron configuration calcium (ca). The electron configuration of calcium is. 1s 2 2s 2 2p 4: Web in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. These atoms have 4s valence electrons, but no d electrons. Since 1s can only hold two electrons the next 2 electrons for chromium go in the 2s orbital.
Calcium Electron Configuration Periodic Table
1s 22s 22p 63s 23p 64s 2. Different configurations can be favoured in different chemical environments. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the calcium atom. Electron configuration.
Electron configuration of calcium 3D scene Mozaik Digital Learning
Web electrons are speed throughout the atom on different levels and subshells. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2# or simply #ca: (2 marks) atom/ion electron configuration (long form only) reasoning for electron configuration difference ca atom ti2 ion 1s 2 2s 2 2p 4: Explain why they do not have the same electron configuration.
Calcium Ca (Element 20) of Periodic Table Elements FlashCards
Electron configuration of oxygen (o) [he] 2s 2 2p 4: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: These atoms have 4s valence electrons, but no d electrons. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. 1s 2 2s 2 2p 4:
Calcium Facts
Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si) phosphorus (p) sulfur (s) chlorine (cl) argon (ar) potassium (k). Electron configuration can be done in two ways. Web this video help for you.how to write the electron configuration calcium (ca). The.
Electron Shell Calcium Electron Configuration Valence Electron PNG
Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web electron configuration of carbon (c) [he] 2s 2 2p 2: In order to write the ca electron configuration we first need to know the number of elect. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Web the electron configuration of calcium.
Electron Configuration Of Calcium (ca) worksheet
Electron configuration through orbit (bohr principle) electron configuration through. Different configurations can be favoured in different chemical environments. How do we write the configuration for ca2+? Explain why they do not have the same electron configuration. Web in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are.
Calcium Electron Configuration YouTube
Web the electron configuration of calcium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 calcium is metal because it is in group 2 which makes it an alkaline earth metal and a powerful reducing agent. And thus for the neutral atom, we have 20 electrons to distribute: Suggest corrections 19 similar questions q. This group.
Calcium Electron Configuration (Ca) with Orbital Diagram
For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Web calcium has an atomic number of 20. Web the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any.
Calcium electron configuration Stock Image C029/5027 Science
Suggest corrections 19 similar questions q. 1s 22s 22p 63s 23p 64s 2. The electron configuration for calcium is: Web the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). And the abridged form is [ar]4s2.
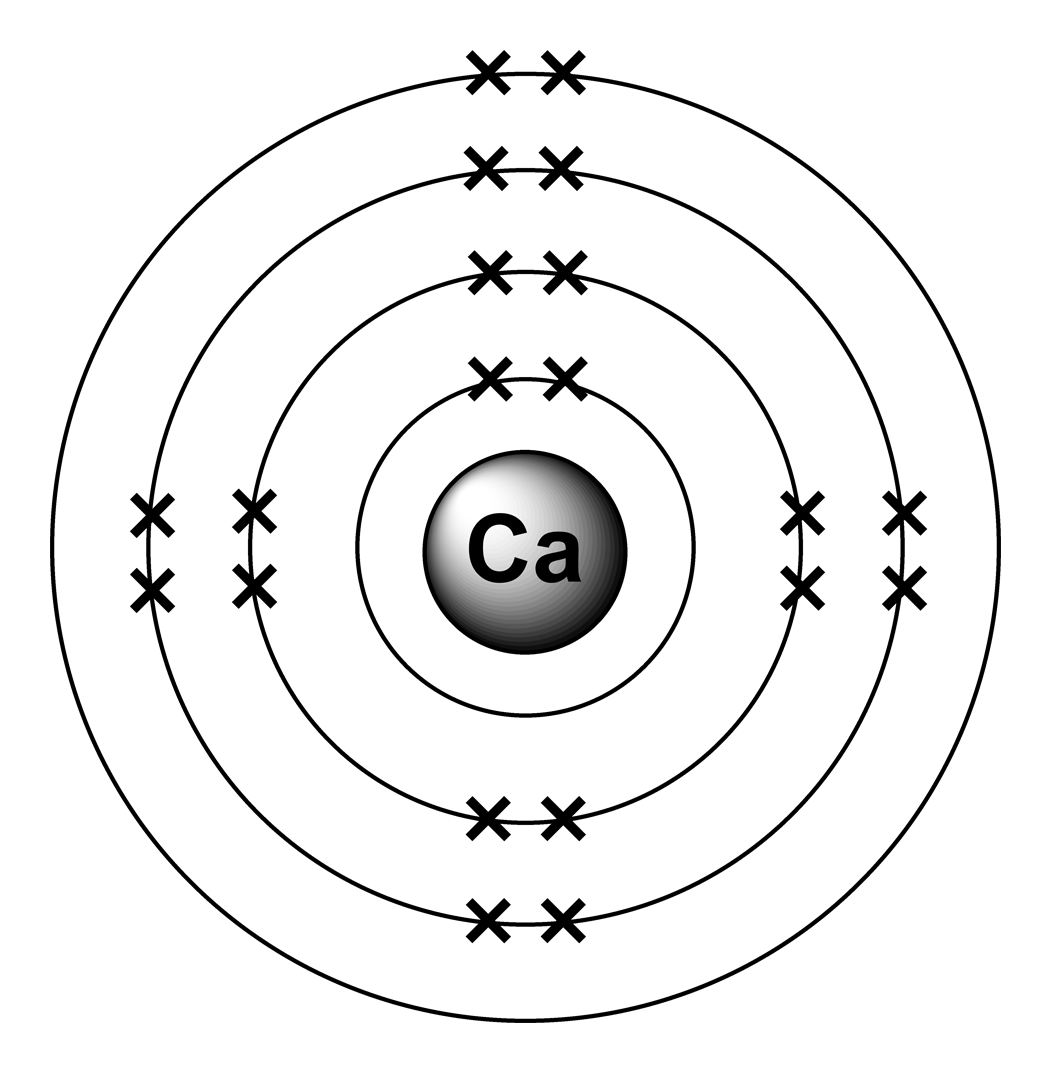
Bohr Diagram Of Calcium
In order to write the ca electron configuration we first need to know the number of elect. And the abridged form is [ar]4s2. Electron configuration through orbit (bohr principle) electron configuration through. Explain why they do not have the same electron configuration. Electron configuration of fluorine (f) [he] 2s 2 2p 5:
Web The Electron Configuration Of Cadmium Ion (Cd 2+) Is 1S 2 2S 2 2P 6 3S 2 3P 6 3D 10 4S 2 4P 6 4D 10.
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2# or simply #ca: Web the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). Electron configuration of oxygen (o) [he] 2s 2 2p 4: Calcium isotopes are 40 ca, 42 ca, 43 ca, 44 ca, 46 ca, and 48 ca.
Different Configurations Can Be Favoured In Different Chemical Environments.
Web wayne breslyn 633k subscribers subscribe 87k views 4 years ago in this video we will write the electron configuration for ca2+, the calcium ion. Web the electronic configuration of calcium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2. The electron configuration for calcium is: Radium pls write in full withoutbridging with the noble gases.
And Thus For The Neutral Atom, We Have 20 Electrons To Distribute:
Web in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Since 1s can only hold two electrons the next 2 electrons for chromium go in the 2s orbital. 1s 22s 22p 63s 23p 64s 2. Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si) phosphorus (p) sulfur (s) chlorine (cl) argon (ar) potassium (k).
These Atoms Have 4S Valence Electrons, But No D Electrons.
The next six electrons will go in the 2p orbital. And the abridged form is [ar]4s2. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web electron configuration of carbon (c) [he] 2s 2 2p 2: